Congenital malformations of the pulmonary vessels include absence or interruption, abnormal sizes, origins, or courses, and abnormal connections of the pulmonary arteries or veins. These abnormalities commonly accompany other congenital pulmonary or cardiac disorders but can occur in isolation. Although some malformations have no clinical significance and may be an incidental finding at imaging, others may have important consequences. For example, pulmonary hypertension may occur in congenital pulmonary artery abnormalities or in anomalies with a left-to-right shunt, such as anomalous pulmonary venous return. In cases in which clinical consequences are absent or mild, recognition and accurate description of congenital abnormalities of the pulmonary vessels are still critical for avoiding misdiagnosis. For example, dysmorphic lung typically seen in interruption of the pulmonary artery can be mistaken for fibrosis or postinfectious scarring.
Radiography may identify a congenital malformation of the pulmonary vessels in a limited number of cases. However, most abnormalities are either first detected or confirmed at computed tomography (CT) or magnetic resonance imaging (MRI), modalities that characterize morphology and identify coexisting abnormalities. An understanding of the typical imaging appearances, associated abnormalities, and clinical manifestations of congenital malformations of the pulmonary vessels is essential in accurate diagnosis of these entities.
Proximal Interruption of the Pulmonary Artery
Embryology, Prevalence, and Epidemiology
This malformation has many names in the literature: unilateral agenesis, atresia, or absence of the pulmonary artery; occult pulmonary artery; and proximal interruption of the pulmonary artery. Interruption of the pulmonary artery is the most appropriate because it describes a break in continuity of the pulmonary arterial tree, beyond which the pulmonary arteries are still present but small because of the reduction in blood flow. Because pulmonary blood flow influences development of the lungs, an interruption in the blood supply in utero results in hypoplasia of the lung. This defect is frequently associated with congenital heart defects, the most common of which are ventricular septal defects and the tetralogy of Fallot. However, other cardiac malformations are also seen, such as coarctation of the aorta, subvalvular aortic stenosis, dextrotransposition or levotransposition of the great arteries, stenosis or aneurysm of the main pulmonary artery, scimitar syndrome, patent ductus arteriosus, and aortopulmonary fistula.
Clinical Presentation
This malformation is usually diagnosed in children with associated congenital heart defects, but isolated cases may not be diagnosed until adulthood. In adults it is usually an isolated phenomenon, occurs more frequently on the right than on the left, and is associated with a contralateral aortic arch in the vast majority of cases. Symptoms may result either from pulmonary arterial hypertension, which is present in 19% to 25% of cases, or from pulmonary infections, which frequently occur in the hypoplastic lung.
Manifestations of the Disease
Radiography
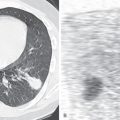
Chest radiography shows the expected findings of a small lung ( Fig. 8.1 ). The degree of lucency of the abnormal side is usually similar to that of the contralateral lung, as air trapping is absent. The hilum on the side of interruption is diminutive, and the contralateral hilum is often enlarged, a finding often best detected on the lateral view (see Fig. 8.1 ). There may be signs of collateral systemic circulation supplying the abnormal lung, such as subpleural reticular opacities, smooth pleural thickening, and unilateral rib notching from enlargement of intercostal arteries. Similar to congenital cardiac diseases associated with pulmonary arterial outflow tract obstruction, there may be parenchymal abnormalities on the side of the interruption that mimic pulmonary fibrosis or scarring from prior infection, such as tuberculosis. Reticulonodular opacities and architectural distortion are common, thought to represent chronic parenchymal damage caused by oligemia.

Ventilation-Perfusion (VQ) Nuclear Imaging
VQ scintigraphy demonstrates complete absence of perfusion and decreased ventilation on the affected side. Delayed washout xenon-133 ventilation scans, which are used to diagnose air trapping, are normal; this contrasts with conditions such as Swyer-James syndrome, in which air trapping is present.
Computed Tomography
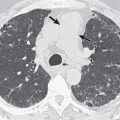
Proximal interruption of the pulmonary artery and associated findings can be definitively diagnosed with CT ( Fig. 8.2 ). CT should be performed in all cases, both to establish a diagnosis and for thorough and precise evaluation of the anomaly, particularly because surgical repair may be contemplated, even in young adults. It is therefore necessary to search for a patent pulmonary artery in the hilum beyond the proximal interruption and to demonstrate its anatomic continuity with smaller proximal and distal pulmonary artery branches. Reduced pulmonary blood flow results in small ipsilateral pulmonary arteries and veins. The systemic arterial system must also be assessed, including the bronchial, phrenic, intercostal, internal mammary, and external mammary arteries, as well as arteries of the pulmonary ligaments. Because embolotherapy of these vessels may be attempted in the event of major hemoptysis, the origin of each artery needs to be carefully analyzed. Systemic anastomoses between the coronary and bronchial arteries may also develop in association with this malformation, for the same reasons that they are seen in chronic thromboembolic disease and other conditions with decreased pulmonary blood flow. The lung parenchyma of the hypoplastic lung or both lungs can demonstrate mosaic attenuation, which may be due to increased collateral perfusion to the abnormal lung, pulmonary hypertension, redistribution of blood flow within the normal lung, or compensatory hyperinflation of the contralateral lung.

On CT indirect signs of systemic collateral circulation include mosaic attenuation, thickened interlobular septa, subpleural reticular opacities or honeycombing, subpleural parenchymal bands, and irregular pleural thickening ( Fig. 8.3 ). All of these findings explain the radiographic appearance of “pseudofibrosis” or “pseudotuberculosis.”

Bronchial thickening is frequent; it is usually from dilated bronchial arteries or recurrent bronchial infections. Cylindrical dilation of proximal segmental or subsegmental bronchi may be a result of the decreased caliber of the accompanying pulmonary arteries, which, because they are enclosed within the same bronchovascular connective tissue sheath, allow greater space for the bronchi to expand. Ischemia of the bronchial walls has also been invoked as an explanation for proximal bronchial dilation, in which the vascular supply of affected bronchi is redistributed toward the peripheral pulmonary arterial circulation. This proximal bronchial dilation is identical to that observed in chronic thromboembolic disease but is completely different from the bronchial dilation in varicose and cystic bronchiectasis.
Magnetic Resonance Imaging
MR angiography accurately depicts this condition and is increasingly used for diagnosis and follow-up imaging. Cardiac MR sequences can also be obtained as part of the same study, allowing characterization of pulmonary artery flow, ventricular function, and associated congenital cardiac abnormalities. Because the condition is most often detected or monitored in childhood or early adulthood, MR angiography has the distinct advantage over CT of avoiding radiation exposure.
Differential Diagnosis
The rarity of this malformation leads to discussion of other possible differential diagnoses, including (1) unilateral postembolic obstruction of a pulmonary artery. Even in the absence of a history of thromboembolism, it is necessary to look for the sequelae of chronic thromboembolic disease at the site of arterial interruption and in the contralateral lung. A purely unilateral manifestation of chronic thromboembolic disease is rare. (2) In Swyer-James-McLeod syndrome, the ipsilateral pulmonary artery is present but small because of decreased perfusion of the affected lung. Findings on VQ scintigraphy are different from those of proximal interruption of a pulmonary artery as discussed earlier. (3) The pulmonary arterial manifestations of Takayasu arteritis could be included in this discussion, but they are usually bilateral, and in most cases the systemic arteries are also involved. The presence of morphologic abnormalities of the pulmonary and systemic arteries evolving at the same time is strongly suggestive of this diagnosis. Takayasu arteritis is more often confused with chronic thromboembolic disease than with proximal interruption of a pulmonary artery. (4) Primary neoplasms of the pulmonary artery and bronchogenic carcinoma are two causes of acquired unilateral obstruction of a pulmonary artery, but both are characterized by the presence of a hilar mass. (5) Fibrosing mediastinitis involving the hilum can also cause unilateral obstruction of a pulmonary artery. This diagnosis should be suspected when there is a pertinent clinical history or other signs of previous granulomatous infection, including a partially calcified mediastinal mass, or stenosis or interruption of other hilar structures, such as bronchi and pulmonary veins. (6) In cases of unilateral stenosis or atresia of the pulmonary veins, the hilar pulmonary artery may appear small and enhanced only in a delayed manner by means of a systemic-to-pulmonary artery retrograde shunt, in which contrast medium reaches the hilar pulmonary artery via retrograde flow. Such a phenomenon can be detected by obtaining a second delayed acquisition focused on the hilum.
Synopsis of Treatment Options
In children and young adults, revascularization of the interrupted pulmonary artery can be attempted by reimplantation or bypass of the affected segment but only when the hilar or proximal intraparenchymal pulmonary artery is patent and suitable for anastomosis. If the caliber of the artery is too narrow, a temporary palliative anastomosis can be attempted to encourage further development of the vessel. When the malformation is not detected until later adulthood, treatment is purely symptomatic. Such treatment may involve embolotherapy of systemic arteries in cases of recurrent hemoptysis or even pneumonectomy to achieve definitive control of hemorrhage.
- •
Rarely discovered in adulthood
- •
Usually occurs as an isolated malformation when first seen in an adult
- •
More common on the right
- •
Clinical findings: pulmonary hypertension, recurrent infection, hemoptysis
- •
Common imaging findings:
- •
Small lung with peripheral reticulation and architectural distortion
- •
Absent or small pulmonary artery at the hilum
- •
Normal pulmonary venous return
- •
Aortic arch usually contralateral to the anomaly
- •
Systemic collateral blood supply
- •
Valvular Pulmonary Stenosis
Embryology, Prevalence, and Epidemiology
Valvular pulmonary stenosis may remain asymptomatic for a long time, depending on the degree of the stenosis, and may not be diagnosed until adulthood, when it may be detected incidentally on chest radiography. Causes of valvular pulmonary stenosis include stenosis secondary to commissural fusion of the pulmonary cusps causing a thickened, dome-like pulmonary valve; a bicuspid pulmonary valve; and valvular dysplasia.
Pathophysiology
Valvular pulmonary stenosis causes a poststenotic systolic jet in the pulmonary trunk. Elevated postsystolic pressure in the right ventricle leads to muscular hypertrophy of the right ventricle, most marked in the outflow tract. In addition, hypertrophy of the right ventricle leads to elongation and reorientation of the right ventricle. These changes in orientation of the infundibulum and pulmonary trunk cause the poststenotic jet to be directed primarily toward the left pulmonary artery.
Clinical Presentation
Valvular pulmonary stenosis may be asymptomatic. It can be associated with fatigue on effort secondary to decreased cardiac output. It can also cause a systolic murmur in the pulmonary outflow tract, as well as electrocardiogram signs of right ventricular hypertrophy.
Manifestations of the Disease
Radiography
The most common radiographic signs are poststenotic dilation of the pulmonary trunk and isolated enlargement of the left pulmonary artery and its proximal branches, with a normal or small right pulmonary artery ( Fig. 8.4 ). These findings can simulate a mediastinal mass or enlarged left hilar lymph nodes. The counterclockwise rotation of the heart can simulate left ventricular hypertrophy. Proximal dilation of the left pulmonary artery may be limited to its upper lobe branches or may extend to involve the left interlobar pulmonary artery, which is easily visible on a lateral view (see Fig. 8.4 ). The repetitive trauma caused by the poststenotic jet can result in focal calcification of the wall of the left pulmonary artery.


Computed Tomography or Magnetic Resonance Imaging
Selective enlargement of the left pulmonary artery is usually present (see Fig. 8.4 ), and right ventricular hypertrophy can be seen. At MRI, cine imaging can show a spin-dephasing flow-void artifact from turbulent blood flow arising from the stenotic valve extending into the main and left pulmonary arteries (see Fig. 8.4 ). The pulmonic valve is moderately thickened, irregular, and dome shaped, and leaflet fusion may be present (see Fig. 8.4 ). In exceptional cases the leaflets may be calcified. In cases of valvular dysplasia the valvular annulus and the main pulmonary artery itself are moderately hypoplastic.
Differential Diagnosis
The differential diagnosis includes other causes of dilation of the main pulmonary artery, such as pulmonary hypertension, idiopathic dilation of the main pulmonary artery, incompetence of the pulmonary valve, intravascular malignancy, and aneurysm. It is also important to use CT to exclude tumors and lymphadenopathy involving the mediastinum or left hilum that may mimic this abnormality on radiography.
Synopsis of Treatment Options
The need for treatment depends on the pressure gradient across the stenosis and its effects on cardiac function. Balloon valvuloplasty has become a primary approach, followed by commissurotomy or “shaving” of thickened leaflets. It may be enough to correct the transvalvular pressure gradient without eliminating the stenosis, thereby effectively exchanging a dome-shaped valve for a bicuspid leaflet. Surgical replacement is used for selected cases, including those refractory to balloon valvuloplasty. Transcatheter bioprosthetic pulmonic valve implantation is now an alternative to surgical replacement at some centers, especially for high-risk patients.
- •
May be detected incidentally in asymptomatic patients
- •
Systolic murmur or abnormal chest radiograph
- •
Common imaging findings:
- •
Dilation of the left pulmonary artery and its proximal branches
- •
Thickening of the pulmonic valve leaflets
- •
Right ventricular hypertrophy
- •
Aberrant Retrotracheal Left Pulmonary Artery (Pulmonary Artery Sling)
Embryology, Prevalence, and Epidemiology
Pulmonary artery sling is thought to be secondary to absence of development, underdevelopment, or resorption of the ventral portion of the sixth left aortic arch or its premature obliteration. As a result, the left pulmonary vascular plexus connects to the sixth right aortic arch. The left pulmonary artery thus develops as a collateral vessel of the right pulmonary artery, from which is derived its anatomic description: anomalous origin of the left pulmonary artery from the right. The great majority of cases are detected in childhood and are associated with other congenital malformations, most commonly involving the tracheobronchial tree and the heart. It is a very rare anomaly in adults, in whom it is often an isolated malformation.
Clinical Presentation
The abnormality is asymptomatic unless associated with congenital tracheal or bronchial stenosis, which may give rise to symptoms of dyspnea or recurrent pulmonary infections.
Manifestations of the Disease
Radiography
The left pulmonary artery originates from the posterior aspect of the right pulmonary artery, courses above the right mainstem bronchus at its origin, and then takes a pathway to the left between the trachea and esophagus before reaching the left hilum. It may be suspected on chest radiographs by the presence of an opacity measuring approximately 2 cm in diameter in the right tracheobronchial angle ( Fig. 8.5 ). Detection of this opacity should prompt consideration of four possible diagnoses: lymphadenopathy, dilation of the azygos arch, partial anomalous pulmonary venous return to the superior vena cava, and a retrotracheal left pulmonary artery. The aberrant vessel can be detected on lateral radiographs when located in the lower retrotracheal region, on which it appears as a round opacity, 10 to 15 mm in diameter, that is indenting the posterior aspect of the trachea. An esophagram demonstrates a smooth extrinsic impression on the anterior esophageal wall.

Computed Tomography
An aberrant left pulmonary artery is well depicted in the transverse plane. The diagnosis is therefore easily made on a CT examination, even without intravenous contrast. The left pulmonary artery passes around the inferior portion of the right and posterior tracheal walls, from which it gets its descriptive name: “pulmonary sling” ( Fig. 8.6 ). Once it reaches the left hilum, the left pulmonary artery adopts a normal intrapulmonary course. Diagnosis by conventional angiography may be difficult because there is almost complete superimposition of the left pulmonary artery on the right pulmonary artery on frontal projections. The aberrant left pulmonary artery may be abnormally small or stenosed. In some cases it may reach the left hilum at a lower position than normal.

Associated tracheobronchial malformations include a tracheal bronchus to the right upper lobe, hypoplasia of the distal trachea ( Fig. 8.7 ), stenosis of the right mainstem bronchus, and complete tracheal cartilaginous rings (napkin rings). Reformatted images are useful for identifying moderate tracheal stenosis ( Fig. 8.8 ). This last malformation has been called the “ring-sling” syndrome; it is characterized by a lack of variation in form and diameter of the cartilaginous tracheal rings between inspiration and expiration. In adults partial calcification of the cartilaginous rings may reveal their abnormal complete character.


Magnetic Resonance Imaging
Although MRI is a very valuable diagnostic examination for arterial malformations, it is inferior to CT for identifying abnormalities of the lung parenchyma and tracheobronchial tree.
Synopsis of Treatment Options
Treatment depends on associated anomalies, particularly of the tracheobronchial tree. A congenital tracheal stenosis can be treated by dilation, stenting, tracheoplasty, or bronchial transplantation. Reimplantation of the left pulmonary artery is not usually attempted in adults.
- •
May be an incidental finding on radiography or CT
- •
May be associated with or result in tracheal or bronchial stenosis
- •
Characteristic imaging findings:
- •
Opacity in the right tracheobronchial angle
- •
Left pulmonary artery arising from the posterior right pulmonary artery
- •
Left pulmonary artery coursing toward the left hilum between the trachea and esophagus
- •
Right Pulmonary Artery–to–Left Atrium Communication
Embryology, Prevalence, and Epidemiology
There is no known embryologic explanation for this malformation. It is almost always located on the right, but it has also been described on the left. More often congenital than posttraumatic, it is extremely rare and may be associated with other malformations, including congenital cardiac malformations and right-sided lobar agenesis.
Clinical Presentation
Besides a right-to-left shunt that may be quite severe, other clinically important complications are the same as those associated with pulmonary arteriovenous malformations: paradoxical emboli and rupture of the aneurysmal sac. On contrast-enhanced echocardiography, microbubbles appear in the left atrium later than occurs with a patent foramen ovale and earlier than seen with pulmonary arteriovenous malformations, generally after two to three cardiac cycles.
Manifestations of the Disease
Radiography
This malformation may be suspected on chest radiography by the association of a right-to-left shunt with a right retrocardiac pseudomass, which corresponds to the aneurysmal sac that forms the direct communication between the right pulmonary artery and the left atrium.
Computed Tomography
Four types of this malformation are recognized. In type I venous return from the right lung is normal, and a vessel unites the posterior aspect of the proximal right pulmonary artery with the left atrium. In type II the supernumerary branch of the right pulmonary artery communicates with adjacent venous structures: the left atrium, the right superior pulmonary vein, or both. The right inferior pulmonary vein is absent. In type III both right pulmonary veins drain into a vascular pocket uniting the right pulmonary artery and the left atrium. In type IV the right inferior pulmonary vein is replaced by three small veins connected to an aneurysmal sac. The aneurysmal sac is adjacent to the right posterolateral aspect of the left atrium. The interlobar right pulmonary artery is dilated and located adjacent to the sac, without interpositioning of the afferent arterial vessels.
Synopsis of Treatment Options
Gianturco coils, Amplatzer occluder devices, ligation, ligation and division, excision, intracardiac repair, and pneumonectomy are all possible options, depending on the angioarchitecture of the malformation.
- •
Very rare
- •
Right-to-left shunt
- •
Major complication is rupture of the aneurysmal sac
- •
Common imaging findings:
- •
Right retrocardiac opacity
- •
Large proximal communication of the right pulmonary artery with the left atrium
- •
Congenital Pulmonary Artery Stenosis
Embryology, Prevalence, and Epidemiology
The embryology of this congenital malformation is unknown. Congenital pulmonary artery stenosis is classified into four types: (1) stenosis of the main pulmonary artery, (2) stenosis of the bifurcation of the main pulmonary artery involving the origin of the left or right pulmonary arteries, (3) multiple peripheral pulmonary arterial stenoses, and (4) stenoses involving both the proximal and peripheral pulmonary arteries.
Pathophysiology
Stenoses that involve short segments and that are proximal in location usually cause poststenotic flow acceleration (a poststenotic “jet”). Stenoses of longer segments are not associated with poststenotic jets. The effects of pulmonary arterial stenosis on the right side of the heart are variable.
Clinical Presentation
Congenital pulmonary artery stenoses are almost always associated with syndromes. Williams-Beuren syndrome, or idiopathic hypercalcemia of infancy, consists of mental retardation, recognizable facial traits, peripheral pulmonary arterial or venous stenoses, and supravalvular aortic stenosis. Other syndromes include Noonan syndrome, Down syndrome, Alagille syndrome, Ehlers-Danlos syndrome, and sequelae of congenital rubella infection. Supravalvular pulmonary arterial stenoses may also be associated with cardiac anomalies, such as the tetralogy of Fallot, transposition of the great vessels, and atrial and ventricular septal defects. In exceptional cases pulmonary arterial stenosis may be isolated.
Manifestations of the Disease
Radiography and Computed Tomography
The radiologic appearance depends on the location of the stenosis, its hemodynamic consequences, and the existence of poststenotic dilation. It is dominated by heterogeneous pulmonary vascularization with hypovascular areas and fusiform poststenotic vascular dilations. Proximal stenoses ( Fig. 8.9 ) of the main pulmonary artery and its bifurcation cause bilateral symmetric hypovascularization. Their effect on the right side of the heart is the same as for valvular pulmonary stenosis.

Differential Diagnosis
The differential diagnosis includes chronic thromboembolic disease, fibrosing mediastinitis, and vasculitides, such as Takayasu arteritis, which may result in similar short-segment stenoses with poststenotic jets, as well as longer-segment stenoses.
Synopsis of Treatment Options
Treatment depends on the hemodynamic consequences of the stenosis. It may involve surgical resection of the stenosis with end-to-end anastomosis or endovascular angioplasty with stenting.
- •
Most commonly occurring in the setting of a syndrome, most frequently Williams-Beuren syndrome
- •
Can be associated with cardiac malformations
- •
Common imaging findings:
- •
Poststenotic dilatation of the pulmonary artery branches
- •
Mosaic attenuation in the lungs
- •
Right ventricular dysfunction
- •
Congenital Stenosis and Atresia of Pulmonary Veins
Embryology, Prevalence, and Epidemiology
This malformation affects one or several pulmonary veins in their extraparenchymal courses, unilaterally or bilaterally. Most often it is discovered during infancy or childhood, rarely in adulthood. There are two types: a localized occlusion secondary to a fibrous ring or a diaphragm, which can be totally obstructive and is located at the venous–left atrial junction, and a more extensive stenosis located at a greater distance from the left atrium, which corresponds to hypoplasia or atresia of the pulmonary vein. Although most investigators agree that stenosis, atresia, and hypoplasia of the pulmonary veins are generally congenital in origin, some have suggested venous thrombosis as an additional possible cause.
Pathophysiology
An understanding of the pathophysiology of a pulmonary venoocclusive syndrome makes it easier to recognize the resultant radiologic signs. This syndrome is associated with an increase in pulmonary vascular resistance in the territory drained by the abnormal veins and redistribution of pulmonary arterial flow to other areas of the lung. The pulmonary artery supplying the venoocclusive territory adapts its caliber according to the reduced blood flow and appears “hypoplastic” yet patent. The resultant oligemia stimulates the development of a collateral systemic-to-pulmonary arterial supply arising from the bronchial and nonbronchial systemic arteries, resulting in a functional systemic-to-pulmonary arterial shunt with retrograde flow within the pulmonary artery. The venous obstruction can cause rerouting of venous blood into the systemic veins, such as the bronchial veins (accounting for the hyperemia of the bronchial walls), transpleural veins, mediastinal veins, periesophageal veins, and the portal venous system. This pulmonary-to-systemic venous shunt explains the “arterialization” and oxygenation of blood in the efferent vessels—namely, the superior or inferior vena cava. The pulmonary venoocclusive syndrome leads to chronic alveolar and interstitial pulmonary edema with thickening of the interlobular septa and peribronchovascular sheets. The chronicity of this edema may lead to pulmonary fibrosis. The lymphatics within the territory of venous occlusion are ectatic, and resorption of alveolar and interstitial fluid via the lymphatics can result in enlargement of the hilar and mediastinal lymph nodes.
Clinical Presentation
The clinical manifestations of stenosis or atresia of the pulmonary veins may be those of an associated congenital cardiac anomaly, pulmonary arterial hypertension, recurrent respiratory tract infections, or hemoptysis. VQ scintigraphy may reveal a “pseudopulmonary emboli” appearance with an unmatched reduction in perfusion or a “pseudopneumonia” appearance if the decrease in perfusion is matched by a decrease in ventilation. Hyperemia of the bronchial walls may be a cause of ventilatory obstruction.
Manifestations of the Disease
Radiography
In cases of unilateral obstruction to pulmonary venous return, the chest radiograph shows a small lung with a small hilum without evidence of bronchial obstruction or air trapping. The absence of air trapping must nevertheless be verified by CT because the venoocclusive syndrome can cause subtle bronchial obstruction secondary to edema and engorgement of the veins and lymphatics within the bronchial walls. In addition, pleural thickening may occur. In cases of bilateral venous obstruction, the radiographic findings are those of postcapillary pulmonary hypertension.
Computed Tomography
The presence of a small lung with a small pulmonary hilum and absence of proximal bronchial obstruction can be verified by CT. The pleura may be thickened, with the formation of transpleural adhesions that facilitate the development of transpleural venous anastomoses. In the lung parenchyma there may be patchy ground-glass opacities and interlobular septal thickening in the venooccluded parts of the lung. The bronchial walls are thickened; at bronchoscopy there is often a “pseudoangiomatous” appearance of the bronchial mucosa. The ipsilateral pulmonary artery is small or may be mistakenly thought to be absent if there is retrograde flow of blood within the artery, creating a hemodynamic obstruction to contrast-enhanced blood flow. Its opacification at CT therefore depends on the timing of image acquisition; it is not opacified in the early arterial phase of contrast administration but enhances later secondary to retrograde flow of contrast medium via collateral systemic-to-pulmonary artery shunts ( Fig. 8.10 ). CT angiography of the hilar region with an early systemic arterial phase, as well as a delayed systemic venous phase, thus provides optimal characterization. The intrapulmonary veins are identifiable, but their juxtaatrial portions are absent. At these sites the left atrial wall appears smooth and does not show the characteristic prominences formed by the pulmonary venous confluence in normal patients. Soft tissue thickening in the ipsilateral hilum and mediastinum may represent the development of a collateral venous circulation bypassing the pulmonary venous occlusion. There may be lymphadenopathy and lymphangiectasia in the ipsilateral hilum and mediastinum secondary to lymphatic engorgement from the pulmonary edema fluid. Signs of venous infarction may also be seen.