CHAPTER 27 Congenital Percutaneous Interventions
ATRIAL SEPTAL DEFECT
Description and Special Anatomic Considerations
Secundum atrial septal defect (ASD), one of the more common congenital heart defects, represents 6% to 10% of all congenital anomalies, occurring in 1 in 1500 live births.1 Secundum ASDs are due to absence, perforation, or deficiency of the septum primum. This defect typically occurs sporadically but has been linked to genetic abnormalities such as Holt-Oram syndrome and mutations on chromosome 5p.
Device closure of an ASD was first performed in 1974 by King and Mills2 with a 24-gauge surgically placed femoral sheath and a double-sided disk device. Technology and technique have been modified and refined over the years; however, the procedure remains conceptually identical. A collapsible double-sided disk device with a metal frame and fabric patches is positioned antegrade through a long femoral sheath (6F to 10F) across the secundum ASD. On extrusion from the sheath, the device expands, creating a patch on both sides of the septum, clamping the rim of tissue surrounding the ASD. The endocardium grows in to cover the device to create a permanent seal. Because of the need for surrounding rim tissue, device closure is limited to secundum-type defects and is not applicable to either primum (no inferior posterior rim) or venosus (no superior rim) ASDs. With recent technologic advances, device closure has rapidly become the treatment of choice for secundum ASDs.
Indications
Device closure is indicated for any size secundum ASD with evidence on echocardiography of right ventricular volume overload. ASD should also be closed in patients with symptoms of exercise intolerance or history of cryptogenic stroke. There is mounting evidence that ASD closure, even in the elderly, can improve maximal oxygen consumption.3 ASDs have been closed by device in small children, including infants; however, the most common timing for elective closure appears to be between 2 and 4 years of age.
Outcomes and Complications
Concurrent controlled trials comparing surgical closure with device closure have shown efficacy rates of more than 96% with significantly lower complication rates and hospital stay.4 Most patients can be discharged on the day of the procedure, with return to full activity within 48 to 72 hours, significantly reducing costs and medical resources.5 Early complications have been minor and occur in fewer than 9% of patients; they consist primarily of transient arrhythmias, vascular injury, and asymptomatic device embolization. Serious complications have been rare but include thrombus formation on the device, heart block requiring pacing, and cardiac perforation.6
Imaging Findings
Preoperative Planning
When an ASD has been diagnosed, complete transthoracic echocardiography should be performed to evaluate the suitability for device closure. This includes specific attention to the pulmonary vein drainage as well as to the size and location of the defect, including tissue rims to the atrioventricular valves, inferior vena cava, right pulmonary veins, aortic valve, and roof of the atrium. If the transthoracic study is inadequate to delineate these structures, OmniPlane transesophageal echocardiography should be performed. Documentation of an adequate atrial septal rim circumferentially (>3 mm, especially at the posterior inferior inlet portion; Fig. 27-1) and evaluation for additional defects, tissue strands, or septal aneurysms with perforations are essential (Fig. 27-2). Identification of all pulmonary veins, particularly the right upper, is essential because of the association of partial anomalous pulmonary venous return with sinus venosus ASD.
There is current interest in the use of three-dimensional echocardiography as well as MRI for the preprocedure evaluation of ASD. Certainly, these modalities improve detection of an anomalous pulmonary vein and give a more complete understanding of the shape of the defect. This may permit more accurate measurement of the long-axis dimension of the defect, which is helpful for choosing the appropriate type and size of device. Although these modalities give additional information, their clinical advantage in typical ASDs is not proved, and they do add to cost and medical service use. In patients with unusual anatomy, they are invaluable (Fig. 27-3).
Procedural imaging for device implantation is a combination of echocardiography and biplane fluoroscopy. In my practice, I use surface echocardiography for implantation in children younger than 6 years (Fig. 27-4) and intracardiac echocardiography for older patients (Fig. 27-5). Transesophageal echocardiography is used in older patients in some institutions; however, it necessitates general anesthesia for the procedure. Balloon sizing of the defect is often done and can be useful to detect multiple defects (Fig. 27-6). There has been experimental animal work with active MRI for device implantation (Fig. 27-7), although there has been no human clinical application to date.
Postoperative Surveillance
The cornerstone of surveillance after implantation remains transthoracic echocardiography. Patients are seen 1, 6, and 12 months after implantation to ensure appropriate device position, to rule out thrombus formation, and to assess right ventricular size and function. In most patients with right ventricular dilation, ventricular size returns to normal by 12 months after implantation. Commonly used devices have different imaging properties by echocardiography and radiography (Fig. 27-8). There have been frame fractures after implantation in certain devices (STARFlex and HELEX); therefore, chest radiography or, if necessary, fluoroscopy is needed at 6 to 12 months of follow-up (Fig. 27-9). MRI (Fig. 27-10) and CT can be used to image septal devices after implantation but have not shown additional clinical utility to date.
PATENT FORAMEN OVALE
Description and Special Anatomic Considerations
Device closure of patent foramen ovale (PFO) was first described in 19877 for the prevention of recurrent stroke associated with paradoxical embolus.8 It has also been used to prevent right-to-left shunting causing desaturation in patients with orthodeoxia-platypnea syndrome.9 The foramen ovale is a flap valve in the atrial septum created by overlap of the superior anterior septum secundum on the inferior posterior septum primum (Fig. 27-11). It is present in all fetuses during development to direct oxygenated venous return from the placenta through the inferior vena cava across the atrial septum, bypassing the right ventricle and unexpanded lungs, to fill the left ventricle, allowing optimal cerebral perfusion. After birth, with redistribution of flow due to lung expansion resulting in an increased left atrial pressure, the PFO closes and seals permanently in 65% to 80% of people, age dependent.10 However, in 20% to 35% of the normal population, the foramen ovale does not fibrose closed and remains patent, allowing unidirectional flow from right to left if right atrial pressure exceeds left atrial pressure. This is physiologically insignificant for most people unless the amount of right-to-left shunting is significant, causing orthodeoxia-platypnea syndrome, or an embolus crosses right to left, resulting in a cryptogenic transient ischemic attack or stroke. Approximately 55% of patients who have had a stroke have a PFO,11 suggesting that it plays an important role in some of these patients.
During the last 14 years, interventional device closure of PFO has become an attractive alternative therapeutic strategy to surgical PFO closure or lifelong anticoagulation for stroke prevention. No controlled comparative studies with these other treatment strategies exist for PFO closure, although several active multicenter protocols in stroke patients are currently comparing device closure with medical therapy for prevention of recurrent stroke. Good comparative data from the ASD literature suggest that the efficacy of device closure of ASD is similar to that of surgical closure, with a significant reduction in complications, hospital stay, recovery time, and medical resource use.4
Outcomes and Complications
Procedural success with PFO device closure is 98% to 100%, with complete closure rates of 51% to 96% at 6 months on evaluation by saline contrast transesophageal echocardiography.12,13 Recurrent neurologic event risk after PFO device closure is 1% to 2% annually, with a 96% 1-year and a 90% to 94% 5-year event-free rate.12,13 These results are significantly influenced by selection of patients because some patients who undergo device closure may have recurrent strokes unrelated to either the PFO or the device. More definitive information about recurrent stroke risk will be available from controlled randomized trials now under way comparing device closure with medical therapy. Procedural complications are uncommon, occurring in fewer than 2% of patients; they include stroke, transient ischemic attack, transient myocardial ischemia (these three due to air or clot embolism with the large delivery sheaths in the left atrium), device malposition or embolization, cardiac perforation with tamponade, and local femoral vein injury.12 Late complications include atrial arrhythmias in 4% of patients, although most are mild and require no treatment,14 and thrombus formation on the device.
Imaging Findings
Preoperative Planning
Because most patients undergo PFO device closure for prevention of stroke recurrence, it is essential to evaluate the patient’s prior neurologic events and to ensure that they were cryptogenic and likely related to the PFO. Stroke associated with paradoxical embolism is a diagnosis of exclusion, so it is imperative to rule out other potential causes of stroke including cerebral aneurysm, carotid or vertebral vessel abnormalities, atrial arrhythmias, left atrial appendage thrombus, cardiomyopathy, and a hypercoagulable state. Standard pre–device closure evaluation includes head and neck MRI or MRA; carotid ultrasonography; saline contrast transesophageal echocardiography with Valsalva maneuver (Fig. 27-12); and hypercoagulable screen, including proteins C and S, antithrombin III, factor V Leiden, prothrombin 20210, methylenetetrahydrofolate reductase (MTHFR), anticardiolipin antibody, and homocysteine. This work-up is essential to help guide decisions about the appropriateness of implanting a device and the optimal medical strategy during the endocardialization process. If there is controversy about the presence or size of the PFO, a saline contrast transcranial Doppler study of the middle cerebral artery with Valsalva maneuver is sensitive,15 allowing quantification of the amount of right-to-left shunting. The test is not specific to a PFO; pulmonary arteriovenous malformations will also result in a positive test result. Because of a small incidence of atrial arrhythmias after device placement, a baseline electrocardiogram should also be obtained.
Procedural imaging includes fluoroscopy with or without echocardiography, most commonly intracardiac imaging. Unlike for an ASD, the angiogram must be performed in the right atrium to demonstrate the right-to-left shunting, best shown on profile in the lateral plane (75 degrees left anterior oblique and 5 degrees caudal; see Fig. 27-11).
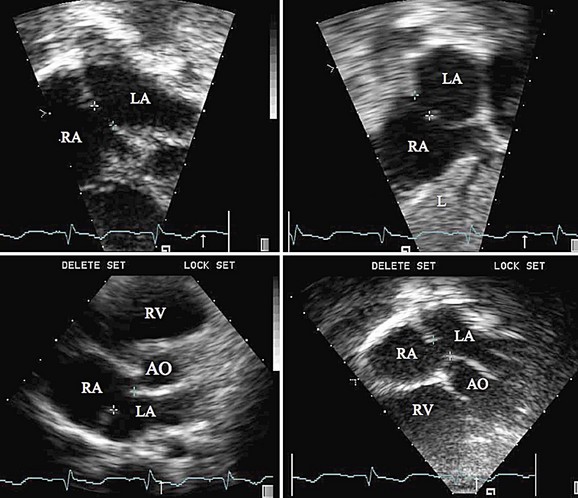
 FIGURE 27-1
FIGURE 27-1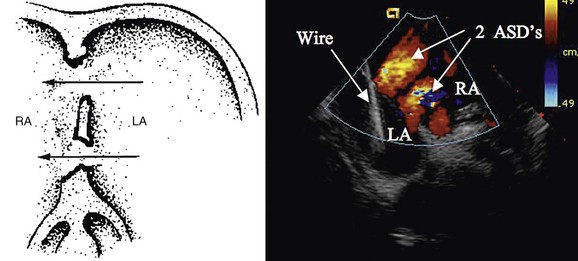
 FIGURE 27-2
FIGURE 27-2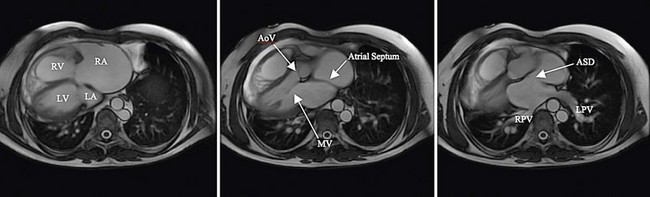
 FIGURE 27-3
FIGURE 27-3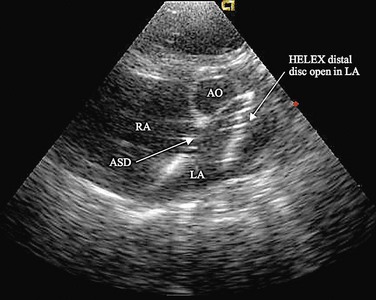
 FIGURE 27-4
FIGURE 27-4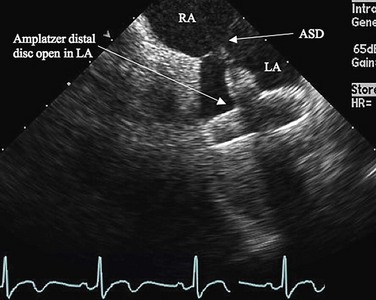
 FIGURE 27-5
FIGURE 27-5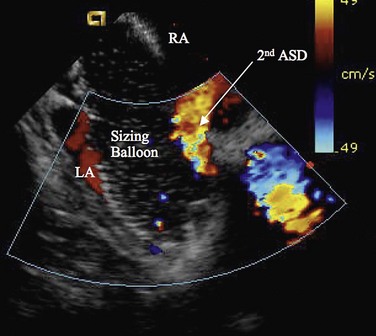
 FIGURE 27-6
FIGURE 27-6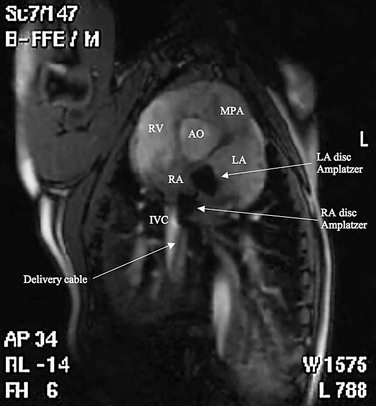
 FIGURE 27-7
FIGURE 27-7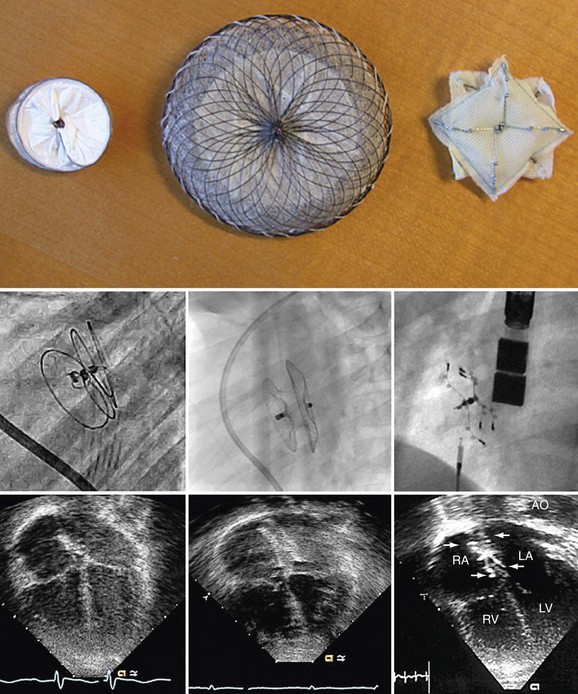
 FIGURE 27-8
FIGURE 27-8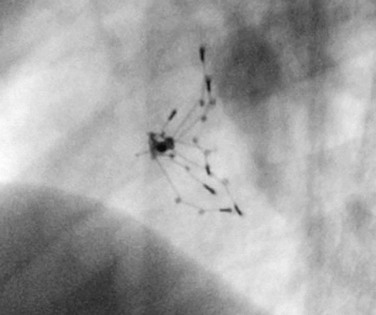
 FIGURE 27-9
FIGURE 27-9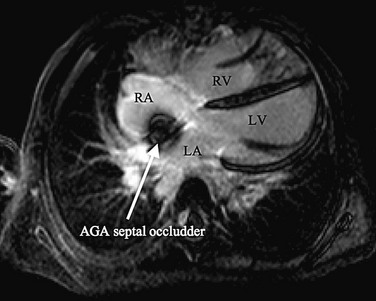
 FIGURE 27-10
FIGURE 27-10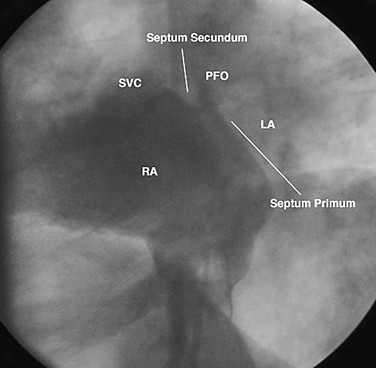
 FIGURE 27-11
FIGURE 27-11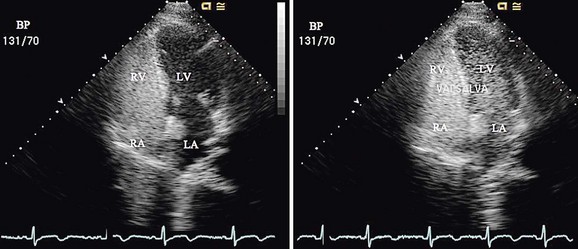
 FIGURE 27-12
FIGURE 27-12


