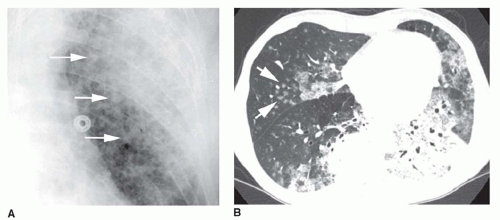
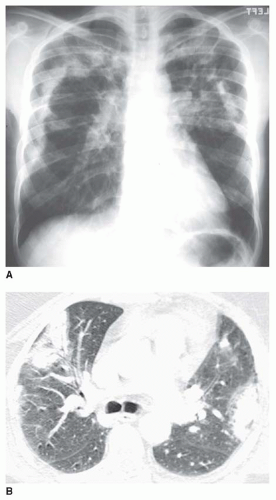
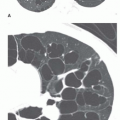
Homogeneous opacity obscuring vessels
Air bronchograms
Ill-defined or fluffy opacities
“Air alveolograms”
Patchy opacities
“Acinar” or air-space nodules
Preserved lung volume
Extension to the pleural surface
“CT angiogram” sign
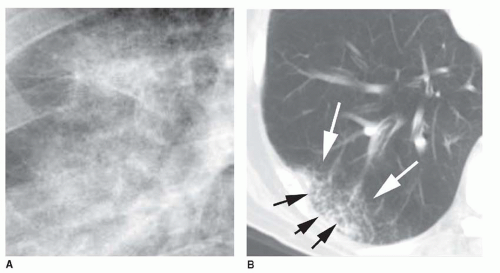
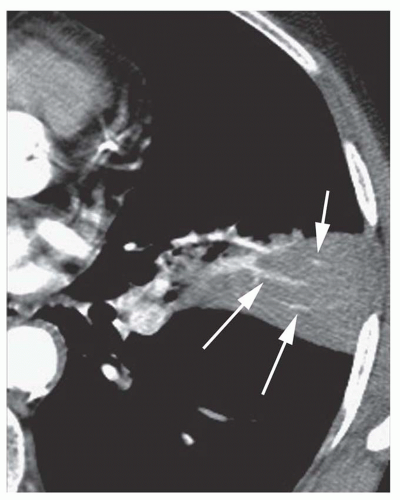
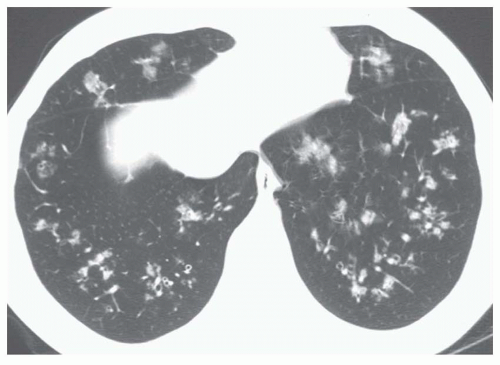
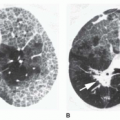
5 to 10 mm in diameter, that occur due to focal consolidation (Fig. 2-5). Although these nodules approximate the size of acini, they tend to be centrilobular and peribronchiolar rather than acinar. They may be seen as the only finding of consolidation or may be seen in association with larger areas of consolidation, usually at the edges of the more abnormal lung.
Water (e.g., the various types of pulmonary edema)
Blood (e.g., pulmonary hemorrhage)
Pus (e.g., pneumonia)
Cells (e.g., bronchioloalveolar carcinoma, lymphoma, eosinophilic pneumonia, organizing pneumonia [bronchiolitis obliterans organizing pneumonia or BOOP], hypersensitivity pneumonitis)
TABLE 2.1 Differential Diagnosis of Diffuse Consolidation
Water (edema) (see Chapter 11)
Hydrostatic (cardiogenic) pulmonary edema
Heart failure
Left atrial or pulmonary venous obstruction
Volume overload
Low intravascular oncotic pressure
Hypoalbuminemia
Liver disease
Renal failure
Increased permeability (noncardiogenic) pulmonary edema
With diffuse alveolar damage (acute respiratory distress syndrome [ARDS])
Acute interstitial pneumonia
Aspiration of gastric acid
Drugs
Fat embolism
Infection and sepsis
Near-drowning
Pneumonia
Radiation
Shock
Toxic fumes or gases
Trauma
Without diffuse alveolar damage
Any cause of ARDS, in a mild form
Drug reactions
Hantavirus pulmonary syndrome
Transfusion reaction
Mixed types of edema
Air embolism
High-altitude pulmonary edema
Neurogenic pulmonary edema
Posttransplantation edema
Postpneumonectomy
Reexpansion edema
Reperfusion edema
Tocolytic therapy
Hydrostatic and permeability edema
Blood (hemorrhage) (see Chapter 19)
Aspiration of blood
Bleeding diathesis
Anticoagulation
Chemotherapy
Leukemia
Low platelets
Collagen-vascular disease and immune complex vasculitis
Systemic lupus erythematosus most common
Behçet’s syndrome
Henoch-Schönlein purpura
Antiphospholipid syndrome
Goodpasture’s syndrome
Idiopathic pulmonary hemosiderosis
Trauma
Vasculitis
Wegener’s granulomatosis
Churg-Strauss granulomatosis
Microscopic polyangiitis
Pus (pneumonia)
Bacterial pneumonia
Pneumonia in an immunosuppressed patient
Tuberculosis
Nontuberculous mycobacteria
Fungal pneumonia (histoplasmosis, aspergillosis most common)
Atypical organisms
Virus
Pneumocystis
Cells
Neoplasm
Bronchioloalveolar carcinoma
Lymphoma and other lymphoproliferative diseases
Eosinophilic pneumonia or other eosinophilic diseases
Organizing pneumonia (BOOP)
Hypersensitivity pneumonitis
Idiopathic interstitial pneumonias
Nonspecific interstitial pneumonia
Desquamative interstitial pneumonia
Sarcoidosis
Other substances
Alveolar proteinosis (lipoprotein)
Lipoid pneumonia (lipid)
Other substances (e.g., lipoprotein in alveolar proteinosis, lipid in lipoid pneumonia).
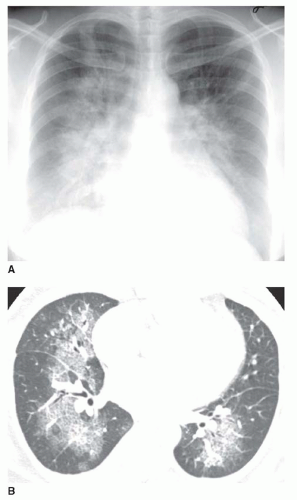
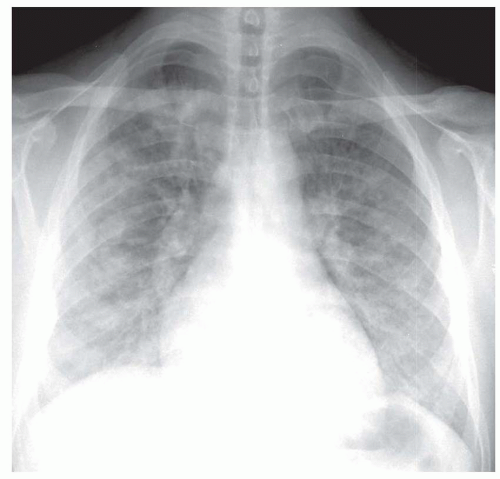
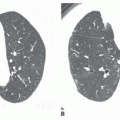
Nonetheless, several patterns of diffuse consolidation may suggest possible causes.
viral pneumonia (cytomegalovirus [CMV], measles), endobronchial spread of bronchioloalveolar carcinoma, pulmonary hemorrhage, or sometimes aspiration.
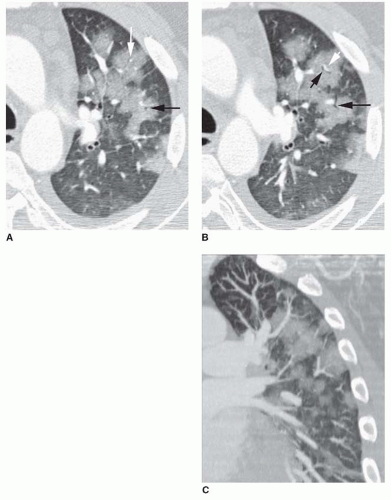
eosinophilic pneumonia; atelectasis; or rarely focal edema. The appearance of focal consolidation may also result from confluent interstitial disease, as in patients with sarcoidosis. The appearance or pattern of focal or multifocal consolidation may be helpful in differential diagnosis.
TABLE 2.2 Differential Diagnosis of Focal Consolidation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
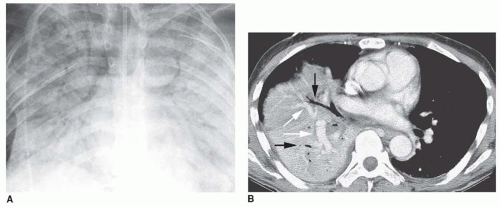
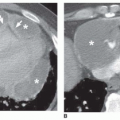
myocardial infarction resulting in papillary muscle rupture and mitral valve prolapse; it occurs because a jet of regurgitant blood is directed into the right superior pulmonary vein. Focal pulmonary hemorrhage may lead to a lobar consolidation. Lobar consolidation is uncommon with pulmonary embolism.
sphere of consolidation as more and more alveoli become involved. As the growing sphere reaches a pleural surface or fissure and cannot spread further, it becomes lobar.
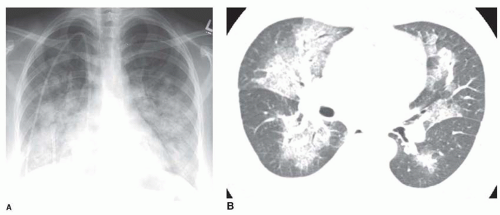
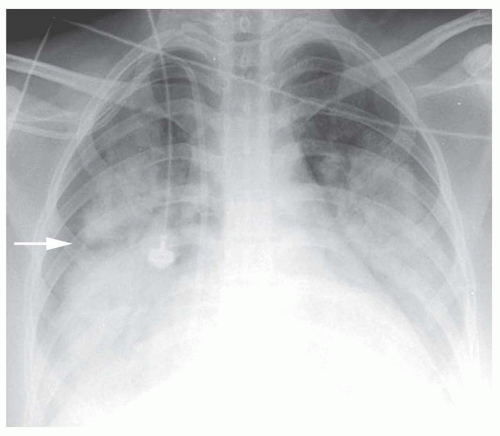
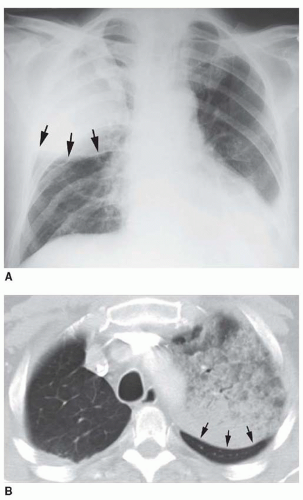
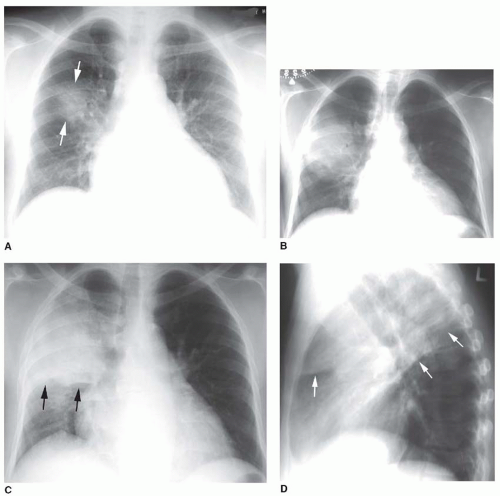
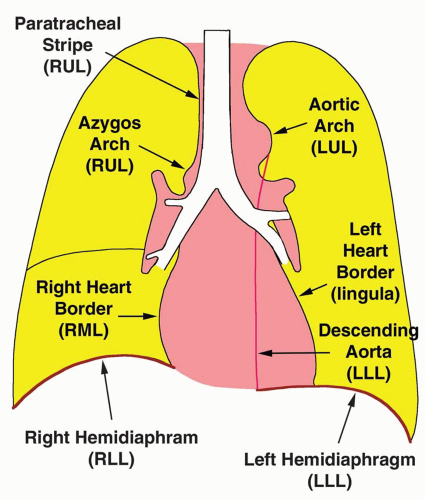
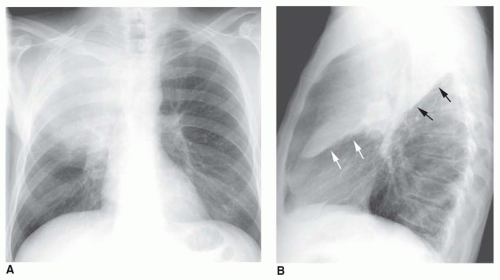
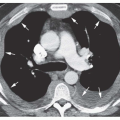
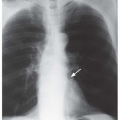
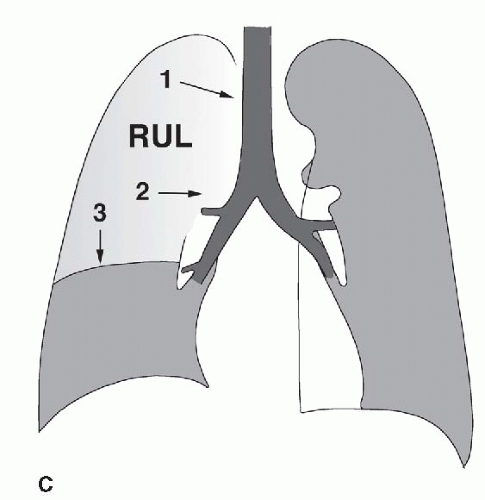 FIG. 2.16. (Continued.) C: Typical findings of right upper lobe consolidation: (1) obscuration of the right superior mediastinum, (2) obscuration of the superior right hilum, and (3) opacity marginated inferiorly by the minor fissure.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|