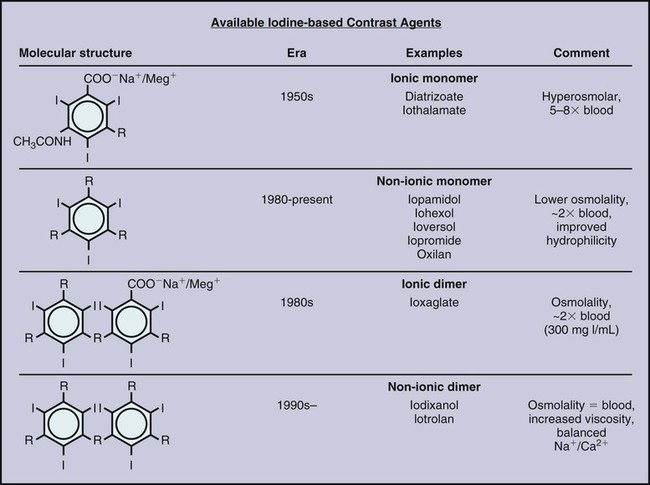
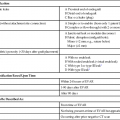
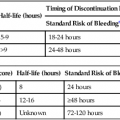
The first reported use of a contrast agent, within a year of the description of x-rays by Roentgen, was for visualization of the vasculature in an amputated hand, highlighting the interest dating from the initial use of imaging in developing contrast agents to aid in defining arteries, veins, and other internal structures.1 Over the next 30 years, the value of contrast agents was widely recognized and investigated. During this period, the major focus was on development of an intravenous (IV) agent for visualization of the urinary tract. Early on it was recognized that iodine was potentially a suitable agent because of its wide availability and its property of blocking x-rays. Organic iodine, however, was soon found to be too toxic to the endothelium to allow parenteral administration. A number of other agents were investigated. From the 1920s to 1950, perhaps the most promising and widely used was a colloidal suspension of thorium dioxide, Thorotrast. This compound was developed for clinical use by Egaz Moniz, a Portuguese scientist, neurosurgeon, and diplomat. He later received the Nobel Prize in Medicine for his development of the now-reviled procedure of prefrontal lobotomy for treatment of various forms of mental illness. Thorotrast was in many ways an ideal contrast agent. It was well tolerated when used for cerebral angiography and provided excellent vascular opacification. It was, however, an alpha emitter and was taken up and retained by the reticuloendothelial system after intravascular administration, thereby causing lifelong radiation exposure. It led to a high incidence of liver and hematologic malignancies as well as cirrhosis and was taken off the market.2,3 Biochemists in collaboration with physicians manufactured a series of compounds that used iodine in various combinations to achieve radiopacity with low levels of toxicity. During this period, toxicity was mainly pain on injection and sclerotic reaction in vessels. Among the investigators in the 1920s were Binz and his students and their collaborator Räth, who developed numerous compounds that were extensively investigated in vitro and in animal studies and even in humans. In the late 1920s, a young American urologist, Moses Swick, working in the laboratory of the eminent German physiologist and physician Professor von Lichtenberg, used several of these compounds. With trial and error, one of them, sodium-2-oxo-5-iodopyridine-N-acetate, was shown to achieve visualization of the urinary tract after IV administration, with limited acceptable endothelial and systemic toxicity. Swick was allowed to present his work, but as was the tradition in Germany at that time, the major credit and first authorship of the resultant paper went to von Lichtenberg.4 Swick’s substantial contributions were not fully recognized until 30 years later, when as an elderly urologist practicing in New York, his pivotal role in the development of intravascular contrast agents was finally publicly recognized and widely lauded.5,6 The compound Swick used consisted of an incompletely substituted four-carbon ring with a single iodine atom attached. Though a marked improvement over previously available agents, it was still somewhat toxic. From the late 1920s through 1954, a number of iodine-based agents were developed and tested in both animal models and the clinical setting. Swick and his collaborator Wallingford were the first to propose the use of a six-carbon (benzene) ring, but the toxicity of this compound, with a single iodine atom attached and not fully substituted, was too great.6 In the early 1950s, three side chains were added to this six-carbon ring, and a second then a third iodine atom. This fully substituted six-carbon ring with three iodine molecules attached and various side chains at the other three positions had markedly decreased toxicity.7 To achieve chemical stability in solution, this was manufactured as a salt. When placed into solution, one of the side chains dissociated into a positively charged cation (usually sodium) and the remaining iodine-containing anion. Consequently, in solution this compound had an osmolality that was twice what it needed to be: for each of the three iodine atoms, two osmotically active particles were present (Fig. 20-1). The next major development came a little over a decade later.8 Torsten Almen, at the time a young Swedish radiologist working as a research fellow in Philadelphia, noted that these high-osmolality ionic contrast agents (i.e., containing a positive and a negative charge) caused pain on intraarterial injection. He observed that if he swam in salt water with his eyes open, the water hurt his eyes. If he swam in fresh water, however, his eyes did not hurt. Almen then reasoned that the pain on injection was related to the high osmolality and to the property of the compound being a salt, with sodium as a dissociating side chain in most molecules. He developed a nondissociating and nonionic (noncharged) compound, metrizamide (Amipaque). In creating this compound, he not only eliminated the charge but also decreased the osmolality of earlier compounds by half, at the same iodine concentration. Metrizamide, however, was not stable in solution. It was available as a lyophilized powder and had to be reconstituted before use. As Almen had predicted, the amount of discomfort encountered with intravascular injection of metrizamide was strikingly less than with previous agents. This was a major step forward, but because of the inconvenience of this formulation and the occurrence of some unexpected central nervous system complications, as well as the cost of producing the compound, extensive efforts were made to develop other more stable low-osmolality agents. Numerous compounds resulted, many of which are still in use. There were two separate pathways, one of which is now essentially of only historical interest. With the aim of lowering the osmolality, two benzene rings were linked together, forming a dimeric compound. The resulting formulation still had a dissociating side chain in solution, but it now had six iodine atoms paired with two osmotically active particles and thus had an osmolality half that of the earlier contrast agents. This compound, ioxaglic acid (Hexabrix [Guerbet, Aulnay-sous-Bois, France]) was developed in France and widely used in the 1980s.9 The other approach, which mimicked the development of metrizamide, was to create monomers of fully substituted benzene rings with three iodine atoms attached but without a dissociating side chain. This is the basic formulation of the vast majority of contrast agents currently in use.10–12 These so-called low-osmolality agents, however, have an osmolality that is still twice that of blood (see Fig. 20-1). All the minor adverse events associated with the earlier higher-osmolality agents, including discomfort, pain, nausea, and vomiting, were markedly decreased with these low-osmolality agents, as were the direct cardiac effects13–16; but the very rare life-threatening reactions still occurred, perhaps at the same rate. It was theorized that both safety and comfort could be further improved if the osmolality could be decreased to that of blood. This was accomplished by creating a nondissociating nonionic dimer. Two such agents were developed in the 1980s. The first, iotrolan, was widely investigated and found to be generally safe and even more comfortable than the nonionic monomers.17 After its release in much of Europe, to a limited extent in the United States, and in Japan, however, it was noted in Japan that there was a relatively high incidence of delayed cutaneous reactions, some of which were severe.18 This led to removal of iotrolan from the market. The second nonionic monomer, iodixanol (Visipaque [GE Health Care, Princeton, N.J.]), was developed around the same time, was released widely in the mid- to late 1990s, and is now fairly widely used.19 It is safe and causes minimal discomfort on injection, as expected, even less than the low-osmolality agents. As noted, all modern iodinated contrast agents consist of one or two fully substituted six-carbon rings (see Fig. 20-1). The conventional high-osmolality ionic contrast agents still exist, with a single benzene ring, three attached iodine atoms, and the property of dissociating into two charged particles in solution. They are very safe and inexpensive but are currently used to a limited extent, usually for injection in small volumes for opacification of collecting systems, as with placement or injection of biliary drains, nephrostomies, or abscess drains. Iothalamate (Conray) is perhaps the most widely used of this class of contrast agents. Various side chains unique to the commercially prepared individual nonionic molecules are attached at the three carbon sites not occupied by an iodine atom. All these so-called low-osmolality agents are nonionic, nondissociating, and stable in solution. All iodinated contrast agents in current use are relatively small molecules with a molecular weight of less than 1 kD. They are therefore too small to act directly as antigens and induce an antigen-antibody (i.e., allergic) reaction. The degree of opacity achieved with contrast agents is a function of a number of variables. The iodine concentration is the most important. All contrast agents are prepared in a stable pH-balanced solution with various additives (e.g., calcium disodium EDTA) to maintain both the pH and stability during storage. In general, the higher the iodine concentration in a contrast agent, the higher the osmolality. For use in angiography, an iodine concentration of around 300 mg/mL is usually sufficient. It is important to keep in mind that an iodine concentration that is too low will not allow sufficient delineation of anatomic abnormalities such as vessel irregularities. On the other hand, if the iodine concentration is too high, there will be insufficient x-ray penetration to define certain structures such as plaque. Other obvious variables in visualization are viscosity of the contrast agent, rate and volume of injection, size of the vessel to be visualized, the patient’s cardiac output, and blood flow in the specific region. Viscosity, which varies largely as a function of the side chains of the compound as well as the iodine concentration, has an effect on how well a bolus is maintained, in addition to how difficult it is to inject the contrast agent. The viscosity of most contrast agents is two to three times that of blood, and hand injection is not difficult. The single currently available nonionic dimer has a viscosity substantially greater than that of the monomers. On the one hand, this may allow the bolus of contrast to remain intact to a greater extent as it is injected into the bloodstream. Conversely, there may be a discernible difference when it is injected by hand through a small catheter. Various technical factors such as the contrast sensitivity of the imaging system and the use of subtraction techniques help define the optimal concentration plus volume of contrast agent that is necessary. If contrast material is injected into a small artery in a patient with low cardiac output and digital subtraction angiography (DSA) is performed, either a low concentration (e.g., 200 mg iodine per milliliter) or a very small volume can be used. On the other hand, if a large structure such as the aorta is to be visualized in a patient with high cardiac output, it is often necessary to use a higher iodine concentration (e.g., 320-360 mg of iodine per milliliter) and to use both a higher flow rate and a larger volume. With IV injection for visualization of vascular structures and organs, the quality of the images is similarly dependent on the aforementioned factors: imaging technology, patient’s size and cardiac output, rate and volume of injection, and iodine concentration. With rapid multislice CT scanning, the iodine concentration, volume of injection, and timing of filming are crucial and still subject to wide debate.20–22 All iodinated contrast agents are essentially immediately distributed throughout the extravascular space. They are not metabolized and are excreted by glomerular filtration without tubular reabsorption. The half-life of contrast agents in general is about 60 minutes. Although there is still some debate, there is minimal if any free iodine in preparations of iodinated contrast agents. In general, therefore, there is no concern about the effect of contrast agents on the thyroid.23,24 It is important to recognize that (1) organic iodine is relatively toxic if injected directly,6 and (2) iodine is an essential element, so a true allergy to iodine is incompatible with life. Contrast agents bind minimally to protein. It is possible certain adverse reactions to contrast agents, as discussed later, are the result of complexes formed by binding to protein, with consequent antigenicity or activation of acute-phase reactants. It is important to reiterate, however, that iodinated contrast agents bind minimally to protein, and no antibodies to contrast agents have ever been found, despite varied extensive studies over decades. Acute life-threatening and even fatal reactions to contrast agents clearly occur25,26 and have been encountered with all contrast agents in current use, but there are two caveats. First, such reactions are rare. From large studies it is clear that the incidence is less than 1 per 100,000 patients.27,28 Secondly, if appropriately treated, almost all reactions resolve. Third, most deaths that occur in association with the administration of a contrast agent are primarily due to underlying disease rather than the contrast agent itself. In large carefully controlled series, most or all fatalities have occurred in patients in whom the effects of the contrast agent were generally incidental or played a small additive role. For example, the vast majority of deaths in one large registry occurred in patients with severe multitrauma, advanced cancer, or end-stage congestive heart failure.27 It is clear, however, that severe acute reactions to contrast agents occur and can be rapidly fatal. It is therefore essential that before a contrast agent is administered, all patients be appropriately screened, and knowledge and expertise to treat severe life-threatening reactions be readily available. Multiple ways of screening patients have been recommended, and as in all clinical settings, it is important to adopt approaches based on the best available data. The basic requirements are that each patient has a targeted history taken (Table 20-1) and that vital signs and assessment of overall status be recorded. Because shellfish contain iodine, it was long believed that patients with shellfish or other seafood allergies would be more likely to experience reactions, particularly severe ones. This turns out to be completely false.27 Although patients with a strong allergic diathesis do have a minor increased risk of having an adverse event associated with a contrast agent,27,28 this relationship is a surprisingly weak one with no evidence that there is an increased incidence of severe reactions.25 Again, it is crucial to remember that severe systemic reactions are not truly “allergic,” although they may be “anaphylactoid”; that is, they appear to be immune-mediated. Similarly, it has been suggested that patients with asthma are at increased risk for systemic reactions to contrast agents. Although this is probably true, available studies suggest that the risk in such patients is mainly worsening bronchospasm, not an alternate severe systemic reaction. This bronchospasm almost invariably responds readily to β-agonist inhalers. TABLE 20-1 Prescreening Questions for Patients Prior to Contrast Administration These caveats notwithstanding, it is clear that the single best predictor of a reaction to an iodinated contrast agent is a previous reaction to such an agent. Again, if these reactions were truly manifestations of an allergy, readministration of a contrast agent would inevitability lead to another reaction, one at least as severe as the initial reaction. This is clearly not the case. From large studies, the incidence of recurrent reactions depends to some extent on the route of administration.27 Another misconception, similar to the discredited belief that patients with shellfish allergies are at particular risk, is that the risk for a systemic reaction is higher with IV than with intraarterial injection. This also is clearly not true. Overall, far from approaching 100%, the risk of recurrent reactions in patients with a documented previous reaction is on the order of 7% with IV administration of contrast, 12% with intraarterial injection, and about 25% with intracoronary injection.27 The reasons for these differences in risk are unclear. They may in fact be dependent in part on erroneously ascribing an adverse event to a contrast agent rather than to a procedure or underlying disease. For example, shortness of breath may be an anxiety response rather than bronchospasm, and hypotension more often occurs as a manifestation of a vasovagal reaction than as a sign of an anaphylactoid response to contrast administration. The important point is that the single best predictor of a reaction to a contrast agent (i.e., a prior contrast reaction) is not a very good predictor at all. Contrast agents are extremely safe. Serious life-threatening reactions, though very rare, occur unpredictably, so it is imperative that patients be appropriately screened and that the knowledge and expertise to treat reactions always be readily available. For a vasovagal reaction, treatment consists of leg elevation (more effectively increases intravascular volume than Trendelenburg position), administration of IV fluids at a high rate, and if necessary, administration of atropine, 0.5 to 1 mg IV. Vagal reactions, in general, are life threatening only if they do not resolve.29 It is therefore crucial to monitor patients until their heart rate and blood pressure return to normal or to baseline levels. An acute anaphylactoid reaction, with hypotension and tachycardia, must be treated as is any cardiovascular collapse. Call a code, continuously assess and record vital signs, make sure there is good intravascular access, infuse fluids such as normal saline (depending on the patient’s cardiac and diabetic status), and if necessary, administer epinephrine. As is rapidly apparent whenever it is administered, epinephrine is an extremely potent α- and β-adrenergic agonist and thus causes marked peripheral vasoconstriction and tachycardia. It should therefore be administered with great caution and only when really necessary, since it can be dangerous in patients with underlying coronary artery disease. Epinephrine is available on code carts in two preparations. Both contain 1 mg (1000 µg), but at a 10-fold difference in concentration: as a 1 : 10,000 mixture in a 10-mL syringe (100 µg/mL) or as a 1 : 1000 mixture in a 1-mL syringe (1000 µg/mL). A 1-mL vial of a 1 : 1000 mixture is also available. The 1 : 1000 preparation is recommended only for subcutaneous administration. In patients with existent or incipient cardiovascular collapse, subcutaneous administration is not appropriate; cutaneous vasoconstriction occurs, and the drug will be slowly and unpredictably absorbed. Administration in an acute life-threatening setting should, if at all possible, be directly IV, using the 1 : 10,000 concentration. When needed, 1 to 2 mL (100-200 µg) should be administered IV as the initial dose. The same dose can be readministered at 2- to 5-minute intervals as needed while keeping in mind that although it should be administered as expeditiously as possible, the dose should also be limited so that as little as necessary is given. Many additional adverse reactions to iodinated contrast agents have been documented, ranging from nausea and vomiting to pain, urticaria, and contrast medium–induced nephropathy (CIN).30 Nausea, vomiting, and pain on injection are largely but not completely related to osmolality. As the osmolality of agents decreases from 1500 to 2000 mOsm/L (with the older high-osmolality ionic agents) to 500 to 700 mOsm/L, the incidence of nausea and vomiting decreases markedly. Both still occur, but infrequently. With isotonic agents, discomfort on injection is essentially absent. Although this is clearly an advantage, routine use of a nonionic dimer is largely obviated at this time due to the added cost. Urticaria is a separate problem. Its occurrence in patients appears to increase the likelihood of recurrent urticaria, but NOT of a subsequent more severe contrast reaction. Urticaria occurs more often than it is diagnosed; if patients are thoroughly and carefully examined for hives, they are often seen in patients who are asymptomatic. If symptomatic, hives generally respond quickly to diphenhydramine (Benadryl, 25-50 mg orally or IV), but this medication can cause drowsiness, so it should only be used for symptomatic relief and only if the patient is not going to drive for the next 6 to 12 hours.30
Contrast Agents
History
Physiology
Adverse Reactions to Iodinated Contrast Agents
General

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Contrast Agents