Chapter 28 Contrast media
Requirements of ‘the ideal’ contrast medium and types of contrast agent
• concentrated in the required area when injected
• rapidly eliminated when necessary
• of appropriate viscosity for administration
Negative contrast media
The following gases create negative contrast on radiographic images:
• Air: Introduced by the patient during a radiographic examination, e.g. inspiration during chest radiography, or can be introduced by the radiographer as part of the examination in a double-contrast barium enema
• Oxygen: Introduced into cavities of the body, for example in the knee during arthrography to demonstrate the knee joint
• Carbon dioxide: Introduced into the gastrointestinal tract in conjunction with a barium sulphate solution to demonstrate the mucosal pattern, e.g. double-contrast barium meal. For the barium meal it is formulated as effervescent powder (e.g. ‘Carbex’ granules) or ready-mixed carbonated barium sulphate (e.g. ‘Baritop’). Carbon dioxide can also be introduced into the colon when performing a double-contrast barium enema. It has been recommended that carbon dioxide be used as the negative contrast agent in a double-contrast barium enema, rather than air, as it causes less immediate abdominal pain1 as well as less post-procedural pain and discomfort.2 However, some studies have shown that carbon dioxide produces inferior distension and additional insufflations are required to maintain adequate quality distension.3 Carbon dioxide can also be used as an alternative contrast to iodinated contrast for diagnostic angiography and vascular interventions in both the arterial and the venous circulation. The gas produces negative contrast owing to its low atomic number and low density compared with adjacent tissues.
Positive contrast media
Barium and iodine solutions are used to create positive contrast on radiographic images.
Barium sulphate solutions (BaSO4) used in gastrointestinal imaging
• High atomic number (56) producing good radiographic contrast
• Excellent coating properties of the gastrointestinal mucosa
Patients rarely have allergic reactions to barium sulphate but may react to the preservatives or additives in the solutions. Barium sulphate preparations are usually safe as long as the gastrointestinal tract is patent and intact. A severe inflammatory reaction may develop if it is extravasated outside the gastrointestinal tract; this is most likely to occur when there is perforation of the tract. If barium sulphate escapes into the peritoneal cavity, inflammation and peritonitis may occur. Escaped barium in the peritoneum causes pain and hypovolaemic shock and, despite treatment which includes fluid replacement therapy, steroids and antibiotics, there is still a 50% mortality rate; of those who survive, 30% will develop peritoneal adhesions and granulomas.4 Aspiration of barium solutions during upper gastrointestinal tract imaging is considered to be relatively harmless, most frequently affecting the elderly patient. Physiotherapy is usually required to drain the aspirated barium and should be performed before the patient leaves the department.
• Suspected partial or complete stenosis
• Haemorrhage in the gastrointestinal tract
• Prior to surgery or endoscopy
• If the patient has had a recent gastrointestinal wide bore biopsy (usually within 3–5 days) or a recent anastomosis
Iodine-based contrast media used in medical imaging and their development
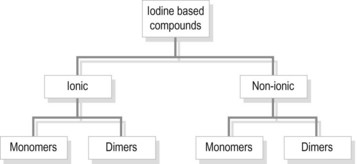
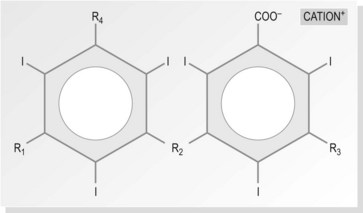
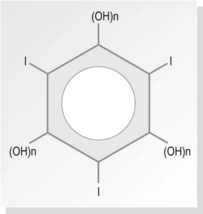
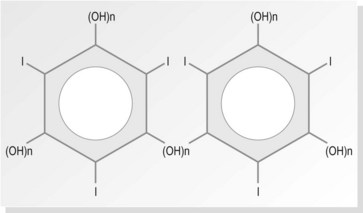
The largest group of contrast media used in imaging departments are the water-soluble organic preparations in which molecules of iodine are the opaque agent. These compounds contain iodine atoms (iodine has an atomic number of 53) bound to a carrier molecule. This holds the iodine in a stable compound and carries it to the organ under examination. The carrier molecules are organic, containing carbon, and are of low toxicity and high stability. Iodine is used as it is relatively safe and the K edge = 32 keV (binding edge of iodine K-shell electron), thus being close to the mean energy of diagnostic X-rays. Selection of kVp for imaging examinations using iodine-based contrast plays a part in providing optimum attenuation. The absorption edge of iodine (35 keV) predicts that 63–77 kVp is the optimal range. The iodine-based compounds are divided into four groups (Fig. 28.1) depending on their molecular structure, as follows:
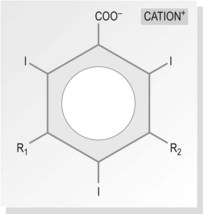
Ionic monomers – high osmolar contrast media (HOCM) (Fig. 28.2)
The basic molecule of all water-soluble iodine-containing contrast media is the benzene ring. Benzene itself is not water soluble; to make it soluble, carboxyl acid (COOH) is added. Three of the hydrogens in this molecule are replaced by iodine, rendering it radio-opaque, but it still remains quite toxic. The remaining two hydrogens (R1 and R2 in Fig. 28.2) are replaced by a short chain of hydrocarbons, making the compound less toxic and more acceptable to the body. The exact nature of these compounds differs between different contrast media, but they are usually prepared as sodium or meglumine salts as these help to provide solubility.
The percentage solution
Percentage iodine content in contrast media
| Contrast media | Percentage solution | Iodine concentration of solution |
|---|---|---|
| Urografin 150 | 30 | 146 mg/mL |
| Urografin 370 | 76 | 370 mg/mL |
| Gastrografin | 76 | 370 mg/mL |
| Niopam 370 | 75.5 | 370 mg/mL |
Essential criteria for the ‘ideal’ intravenous contrast agent
• Heat/chemical/storage stability
• Available at the right viscosity and density
• Low viscosity, making them easy to administer
• Persistent enough in the area of interest to allow its visualisation
• Selective excretion by the patient when the examination is complete
• Same osmolarity as plasma or lower
• Non-toxic, both locally and systemically
Possible side-effects of ionic-based contrast media
Primary effect – image contrast
• Normal blood plasma ~300 mOsm/kg water
• Ionic monomer ~1200–2400 mOsm/kg water, making it very hypertonic
• Ionic dimers, and non-ionic monomers and dimers (LOCM) are still hypertonic but to a much lesser degree, reducing the osmotic activity. They are, however, more expensive. Isotonic iodixanol (Visipaque) has approximately a third the osmolality of the non-ionic media and a sixth of that of the monomeric ionic media.
Secondary effect – adverse events
1 Idiosyncratic reactions are dose dependent and usually anaphylactoid in nature. These are unpredictable, having a prevalence of 1–2% (0.04–0.22% severe), and are fatal in 1 in 170 000.5
2 Non-idiosyncratic reactions are divided into chemotoxic and osmotoxic. Chemotoxic effects can be minimised through the use of LOCM. As LOCM are available at a reasonable cost the use of higher-toxicity substances could be challenged medicolegally.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree