Central endobronchial lesions
– Forceps biopsy
– Brush
– Bronchial washing
– Transbronchial needle aspiration
Peripheral pulmonary lesions
– Forceps biopsy
– Brush
– Curette
– Bronchoalveolar lavage
– Transbronchial needle aspiration
Hilar-mediastinal lesions
– Cytology transbronchial needle aspiration
– Histology transbronchial needle aspiration
Indications, technique, possibilities and limits of each sampling method (forceps biopsy, brushing, bronchial washing, transbronchial needle aspiration) and of its association are reported.
Whenever a biopsy technique is employed, its use must always be guided by a global clinical assessment of the patient, evaluating the risk/advantage ratio and the benefits that can be obtained by the procedure case by case.
Only by integrating clinical, imaging and bronchoscopic techniques will it be possible to optimise bronchoscopy, thereby obtaining the best diagnostic accuracy, minimising the costs involved and having the lowest incidence of risks.
Introduction
One of the main goals of diagnostic bronchoscopy is to obtain a cytohistological assessment of bronchial, pulmonary and hilar-mediastinal lesions. In order to attain this objective, several sampling instruments can be used through the flexible bronchoscope, depending on the morphology and location of the pathological process. The term “conventional biopsy techniques” implies all those traditional sampling techniques that can be used without adopting the latest technology. In this chapter, conventional biopsy techniques will be divided on the basis of the location of the lesion, analysing methods used for sampling central endobronchial lesions, peripheral pulmonary lesions or lung parenchyma and the pathological processes of the hilar-mediastinal area (Table 15.1).
Central Endobronchial Lesions
Central endobronchial lesions (i.e. lesions located within the visible range of flexible bronchoscope) can be approached for sampling cytohistological material, using forceps biopsy, brushing, washing or transbronchial needles.
Forceps biopsy is the most frequently used sampling instrument by bronchoscopists in airway lesions that can be endoscopically visualised. Different sizes and kinds of forceps are currently available on the market: with a cutting or serrated edge (alligator), fenestrated (to reduce tissue-crushing artefacts), with elliptically or spherical-shaped cups and with a needle between the cups (to prevent slippage of the forceps in case of lesions located on the side tracheal or bronchial wall) (Fig. 15.1). There are no comparative studies that clearly demonstrate the advantages of one kind of forceps over the others for sampling central endobronchial lesions, and even the role of forceps size on the diagnostic yield of bronchoscopically visible tracheobronchial tumours has not been evaluated. Therefore, the choice of forceps is generally based on the operator’s experience and preference. Recently, a new commercially available electrocautery biopsy forceps (“hot forceps”) was used for sampling endobronchial lesions, with the aim to prevent bleeding. Even if “hot forceps” do not have a negative impact on the quality of specimens, there is no evidence that they may reduce the incidence of clinically relevant bleeding episodes, and their routine use is not warranted.

Fig. 15.1
Different kinds of forceps biopsy. (a–b) Different size alligators (serrated edge); (c) elliptical-shaped cups with cutting edge; (d–e) fenestrated cups with cutting edge; (f) with a needle between the cups
In order to perform a biopsy of an endobronchial lesion, the forceps must be opened just above the lesion, advanced and pushed towards the area to be sampled, firmly closed and then withdrawn through the working channel of the bronchoscope. The forceps should not be kept too far from the tip of the bronchoscope since, in this way, it is difficult to apply pressure and to maintain contact between the cups and the lesion (Fig. 15.2).
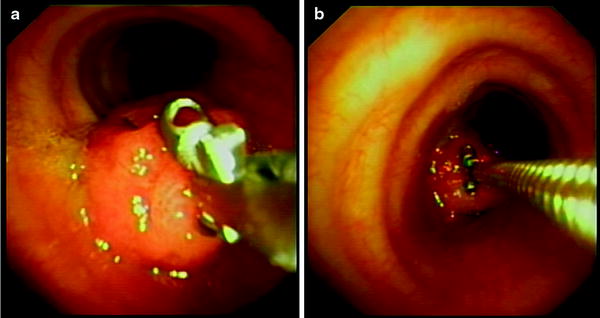

Fig. 15.2
Biopsy of a polypoid lesion obstructing the orifice of the left main bronchus. (a) Correct position: the cups are open and the forceps are pushed towards the lesion. (b) Wrong position: the forceps are kept too far from the tip of the bronchoscope
The major advantage of forceps biopsy is the possibility of obtaining specimens suitable for a histological evaluation, while the limitation of this tool is the difficulty in sampling submucosal or peribronchial lesions or in retrieving diagnostic tissue from lesions with a large necrotic component.
When necrotic tumours are encountered, multiple biopsies should be carried out until surface bleeding is visible and viable tissue is obtained.
In central airway lesions, forceps biopsy yields sensitivity that ranges from 74% to 80%, based on the results of two meta-analyses, but there have been studies reporting values higher than 90%. It has been shown that the best diagnostic yield can be obtained by performing three or four biopsies and that the sensitivity does not significantly increase even if more samples are obtained. To improve the diagnostic yield of forceps biopsy, some alternative methods to treat the sample were proposed, such as smearing the biopsy on a slide (imprint cytology) or cytologically examining the biopsy rinse fluid, but these techniques have not been validated and their use is not recommended.
Bleeding is the most common complication of bronchial biopsy. Generally, bleeding is mild or moderate, and its spontaneous resolution occurs in most instances. Even though it is rare, however, bronchoscopic biopsy-induced bleeding could be life threatening when vascular lesions are approached. Risk factors predisposing to this complication may be related to a variety of coexisting conditions inducing coagulation disorders and/or platelet dysfunction, either as a consequence of underlying systemic disorders (haemorrhagic diathesis, uraemia, haemopathies, liver diseases, immunosuppression) or secondary to medications (anticoagulant therapy, clopidogrel, chemotherapeutic agents). The identification of risk factors and, when possible, their correction is the first step to prevent bleeding. Prebronchoscopy routine coagulation screening is unnecessary in patients with no risk factors, but it should be performed in those with known or clinically suspected risks. Bronchial biopsies cannot be performed if platelet count is <50,000/mm3, and these patients should receive six to ten packs of platelet transfusion before bronchoscopy. Oral anticoagulants should be stopped at least 3 days before bronchoscopy, or low-dose vitamin K should be administered to reduce the international normalised ratio (INR) to <2.5. The use of clopidogrel should be stopped 7 days before bronchoscopy. The risk of bleeding may be also related to the nature of the lesion. Some tumours, like carcinoids and endobronchial renal metastases, are hypervascularised and more prone to bleed, but this is not a contraindication to biopsy.
In any case, bronchoscopists should be trained to manage major bleeding when a bronchial biopsy is performed. The first manoeuvre is to rotate the patient in a lateral decubitus with the bleeding side down. This is a simple and easy procedure that could be life saving, since it avoids the inundation of the contralateral lung. Following the positioning of the patient on the lateral decubitus, the bronchoscope must be kept in site, and continuous suction should be applied to prevent spilling of blood to distal airways, avoiding to keep the tip of the instrument too close to the bleeding lesion. Even if there are no controlled studies that demonstrate the real efficacy of the instillation of ice-cold saline and of epinephrine (diluted in a 1:10,000 mixture of normal saline and administered in 2–3 ml aliquots to a maximum of three doses), all the bronchoscopists agree that these manoeuvres may reduce bleeding and that they should be applied. If the source of bleeding is visible and well localised, the use of low-energy laser or of electrocautery with argon plasma may be helpful. If such procedures are ineffective and a major bleeding continues, the intubation of the patient should be considered using a rigid bronchoscope (if it is available and if there is skill and expertise to its use) or a large channel endotracheal tube, to allow the passage of the flexible bronchoscope. The persistence of bleeding may induce to perform a selective intubation of the contralateral main bronchus to isolate the non-bleeding side, using a normal tube or a Carlens device. Bronchial artery embolisation and open surgical procedures could be considered as a last resort, if all the above-mentioned procedures have failed to stop the haemorrhage.
Brushing is a sampling technique which is widely used by bronchoscopists to collect cytological material from bronchoscopically visible lesions of the airways. There are brushes of different sizes (Fig. 15.3), with or without a plastic sheath, but no differences in diagnostic yield have been demonstrated using different types of brushes. Even if disposable or reusable brushes are available on the market, it is recommended to use disposable tools in order to reduce the risk of contamination or cross infection. Some authors suggest that better cytological material can be obtained when the unsheathed brush and bronchoscope are withdrawn together in order to reduce the loss of material during the passage of the brush in the working channel of the instrument, but other studies have not found any statistically significant improvement in diagnostic sensitivity using this technique.
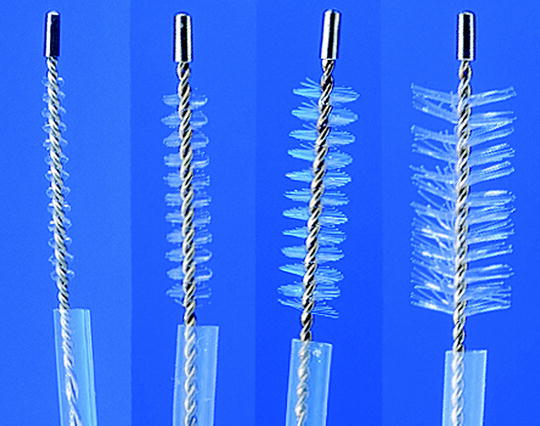

Fig. 15.3
Brushes with plastic sheath of different sizes
Material obtained by brushing can be processed by directly smearing the brush onto a glass slide or by inserting the brush into a saline solution and removing the cells by shaking it vigorously. There are no studies that demonstrate the real advantage of one processing technique over the other.
The limitation of brushing consists in being able to sample only cytological material and only from the superficial layer of the mucosa. As such, this type of instrument is not indicated for submucosal or intraparietal lesions. The advantage of brushing compared to performing a biopsy could consist in obtaining cells from a larger area of the mucosa. The average diagnostic yield from brushing is lower compared to forceps biopsy, with a sensitivity ranging from 59% to 72%. Several authors affirm that by using brushing and biopsy together, diagnostic sensitivity increases and in about 8% of the cases, brushing alone is diagnostic. However, these results are generally reported by non-randomised retrospective studies, performed when the histological characterisation of non-small cell lung cancer was not necessary for therapeutical purposes. In an era of personalised therapy for non-small cell lung cancer, it is important to distinguish between squamous and adenocarcinoma, and it is necessary to obtain an adequate amount of cells or tissues to perform molecular markers-based assessment for guiding therapeutical strategies. Furthermore, while in the past the use of reusable brush did not greatly influence the cost of the procedure, the employment of disposable brush that is recommended today to reduce infection risks could have an economical impact that should be taken into consideration while evaluating the cost-effectiveness of the procedure. In fact, there have been no controlled studies recently that demonstrate the real efficacy of a routine use of biopsy and brushing together in central endobronchial lesions that involve the mucosa. We agree with some authors who hypothesise that the majority of cases which involve the mucosa, where cytology is positive but biopsy is not diagnostic, are a consequence of a non-optimal biopsy sampling technique (wrong forceps positioning, crushing artefacts, necrotic tissue).
Complications resulting from the brushing of central bronchial lesions are very rare. Bleeding and breaking of the brush in the airways have been reported mainly when a reused brush is used.
Bronchial washing is another widely used conventional means of sampling cells from central airway lesions. It can be easily performed by instillation through the working channel of the bronchoscope of about 20 ml of saline that is retrieved by suction. There has been a controversy concerning the optimal timing of washing, when done in association with biopsy (i.e. before or after biopsy), but a more recent prospective study was unable to find any difference in the diagnostic yield for washing before or after biopsies or brushing. The limitation of washing consists in obtaining a sample just by exfoliating superficial cells of the mucosa. The sensitivity of washing for central lung cancer is lower than that obtained by biopsy or brushing, ranging from 29% to 76%, with an average value of 47%. In studies where biopsy, brushing and washing were utilised together, the number of patients diagnosed by washing alone was very small (2.2–3.9%), making the value of the routine use of washing in central bronchial lesions questionable. Since the cost of washing is mainly related to the processing and evaluation of specimens, some authors suggest to collect the washing fluid during bronchoscopy and to hold it in the laboratory, examining the sample only if the other specimens are not diagnostic.
The samples collected by brushing and washing can also be submitted for microbiological evaluation in the suspect of infectious conditions. For this purpose, brush should be agitated in a sterile medium, and washing must be collected in a sterile specimen trap. Even if these specimens are contaminated by oropharyngeal flora during transnasal or transoral passage of the bronchoscope, the value of brushing and washing in the diagnosis of infections has been validated by several studies both in the immunocompetent or immunocompromised patients. The material obtained can be evaluated for bacterial, fungal or mycobacterial smears and cultures. The sensitivity of brushing and washing for the diagnosis of mycobacterial diseases is very high, and it has been reported to range from 58% to 96%. However, routine bronchoscopic samplings for mycobacterial organisms on all patients undergoing bronchoscopy are not recommended in areas not endemic to the disease.
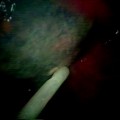
Among the conventional biopsy techniques employed for sampling central bronchial lesions, transbronchial needle aspiration (TBNA) must also be included. The transbronchial use of flexible needles has been introduced for the bronchoscopic approach to hilar-mediastinal lymph nodes located outside the tracheobronchial wall, but this device has also been used for sampling peripheral nodules and central airway lesions (Fig. 15.4). The main advantage of TBNA in bronchoscopically visible lesions is the possibility of the needle to penetrate the deep layers of the mucosa and the peribronchial area, allowing to sample even pathological processes with the intraparietal or submucosal component (Fig. 15.5). Other advantages of TBNA are the following: (1) lower traumatic effects and decreased risks of bleeding, especially for highly vascularised tissue; (2) possibility to sample material from infiltrative lesions covered by hard mucosa, where it could be difficult to obtain specimens with forceps biopsy; (3) better possibility to sample diagnostic cells from highly necrotic lesions, where the needle, bypassing the necrotic component, could collect vital cells from the deeper part of the process (Fig. 15.5) and (4) capability to precisely define the point of sampling, as some time is required during the presurgical staging of lung cancer, in order to accurately define the limit of the tumour. The major disadvantage of TBNA is the price of the needle. Being disposable, it significantly increases the entire cost of the procedure.
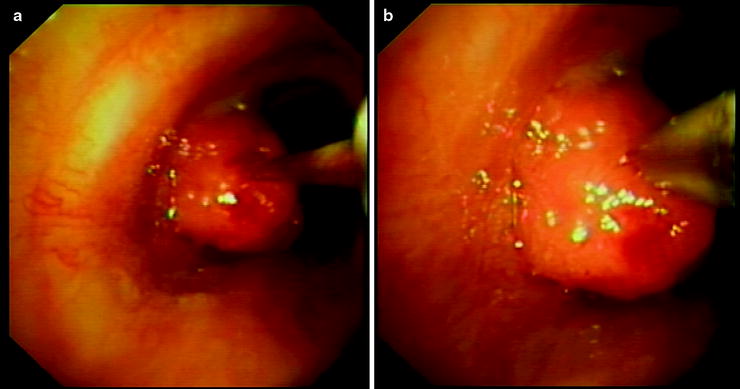


Fig. 15.4
Transbronchial needle aspiration of a central endobronchial lesion. (a) The needle is pointed at the lesion; (b) the needle penetrates the lesion up to the hub of the sheath

Fig. 15.5
Schematic representation of a necrotic lesion with prevalent submucosal component. The needle is able to penetrate the deep layers of the mucosa and has a better possibility of sampling diagnostic material
The sensitivity of TBNA for central bronchial lesions is reported to range from 68% to 91%, with values similar to those obtained by forceps biopsy. Several studies have shown that the use of TBNA in addition to other sampling techniques for central lesions significantly increases the diagnostic yield of bronchoscopy and that TBNA may be the only diagnostic sampling instrument in a percentage of cases that in some studies approaches 20%. This is particularly evident in studies where cases with submucosal-peribronchial lesions have been analysed. In fact, the use of forceps biopsy together with TBNA seems to be the most appropriate integration for sampling central bronchial lesions which allows to obtain specimens at different levels of the bronchial wall, from the surface to deeper layers, to the submucosa and peribronchial area. However, prospective studies that analyse the cost-effectiveness of this association on a large series of patients are lacking, and there is no evidence that justifies the increase in costs due to the routine use of TBNA along with biopsy in all central airway lesions. In our practice, the use of TBNA is limited to the following: (a) cases with a bronchoscopic pattern suggesting submucosal or peribronchial involvement; (b) cases when the macroscopic appearance of the lesion suggests a very vascularised tissue, to test the bleeding risk before performing biopsy; (c) cases where there is a large necrotic component, and the macroscopic pattern of the biopsy shows white samples suggesting necrotic tissue and (d) repeated bronchoscopy, after a first negative bioptic procedure.
In conclusion, conventional biopsy techniques provide a high diagnostic yield in central airway lesions, and they must be considered the gold standard for diagnosis when an endobronchial pathology visible by bronchoscopy is present. Biopsy forceps provide better sensitivity compared to other sampling techniques when the pattern of the lesion suggests mucosal involvement. TBNA should be the preferred sampling instrument when there is evidence of a submucosal or peribronchial spread of the pathology and when the superficial layers of the mucosa may not be involved or when the lesion is very necrotic. Adding brushing to biopsy or TBNA might improve diagnostic yield, but more prospective studies are necessary to evaluate the cost-effectiveness of its use. Washing alone is of little value in the diagnosis of central airway tumours. It may improve the diagnostic sensitivity of bronchoscopy by a small percentage when used together with other sampling instruments, but its routine use is not recommended. Considering the new targeted therapies for lung cancer, future studies should also evaluate the capability of different techniques in differentiating the tumour histotype and in sampling material which is adequate for molecular assessment.
Peripheral Pulmonary Lesions
Different biopsy instruments can be inserted through the working channel of the flexible bronchoscope and pushed into the peripheral airways for sampling cytohistological material from pulmonary lesions located outside the visible range of the bronchoscope (Table 15.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree