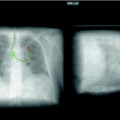
Fig. 54.5
An AP supine CXR demonstrating a pseudopneumothorax, due to a skin flap (arrows). This was confirmed on a subsequent image
There are two types of fissure: standard (oblique and horizontal) and accessory. Standard (interlobar) fissures vary from complete lobar separation to incomplete, occasionally being only a few centimeters in length. Alterations in the course and position of these fissures may be suggestive of underlying pleural or parenchymal disease. The fissures are identifiable on all MSCT scans; the horizontal fissure, separating the right upper from middle lobe, is seen on PA and lateral CXRs, but the oblique fissures are normally only seen on a lateral CXR. Any pulmonary segment may be partially or completely separated from adjacent segments by accessory fissures, uncommonly identified on a PA CXR, but identifiable in up to 20% of CT scans.
Pneumothorax
The presence of gas between the visceral and parietal pleura, a pneumothorax, is always abnormal. Identification of the thin linear visceral pleura with absent lung markings beyond it and increased peripheral radiolucency are the primary radiographic signs but may not always be visible on CXR dependent on the quality of the radiograph and the size of the pneumothorax (Fig. 54.2).
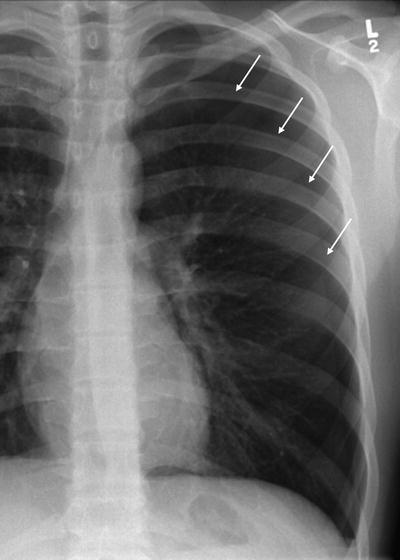

Fig. 54.2
Easily identified pneumothorax, with visceral pleural surface arrowed
Small pneumothoraces are less readily detected on supine or poor quality AP CXRs and in patients with severe pulmonary disease such as bullous emphysema (Fig. 54.3) or confounding radiographic abnormalities such as surgical emphysema. The detection of even small pneumothoraces may in these patients be critical, as they may be the cause of substantial respiratory impairment. On a supine CXR, the presence of a clearly marginated, well-defined hemidiaphragm is suggestive of a pneumothorax, because the gas collects anteriorly in a nondependent position and delineates the anterior aspect of the diaphragm. Detection may be helped by the presence of adjacent collapsed or consolidated lung, allowing the pneumothorax to be more readily detected, but in patients only suitable for supine CXRs a high index of suspicion is required to review the area adjacent to the diaphragm. Expiratory CXRs were previously advocated for the detection of small pneumothoraces but have been shown to add little to the diagnostic yield and are no longer recommended. Lateral CXRs were also previously performed to aid diagnosis, as were lateral decubitus views, particularly in emergency departments and in ICUs, but the use of US and/or CT has for the most part decreased the use of these views.
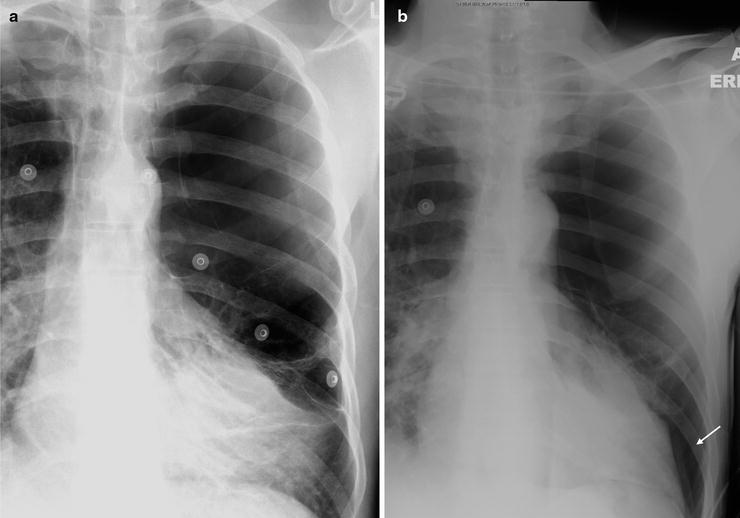

Fig. 54.3
(a, b) The initial CXR demonstrates bullous lung disease on the left, but lung markings are seen at the base. The second CXR shows absent lung markings confirming a pneumothorax
Larger volume pneumothoraces are more easily detected on PA, AP, and supine CXRs and may be of sufficient size to cause complete pulmonary collapse, mediastinal shift if under tension, and the deep sulcus sign in supine patients, with the lateral costophrenic recess seen more caudal than is usually apparent and of decreased radiolucency (Fig. 54.4).
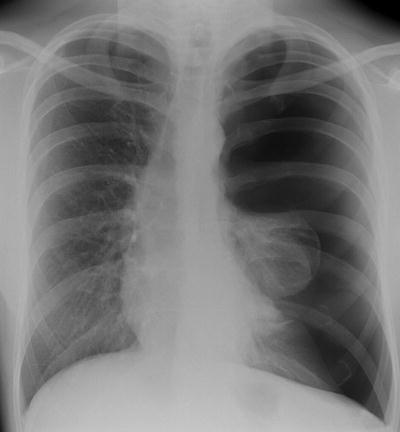

Fig. 54.4
A large left-sided pneumothorax demonstrating mediastinal shift and the deep sulcus sign
Extrapleural artifacts such as skin folds, normal anatomic structures (companion shadows at the apex and medial edge of the scapula), breathing apparatus (oxygen reservoir bag), and other lines, tubes, and wrinkles in clothing or bedsheets are common pitfalls that mimic the visceral pleural edge of a pneumothorax and should be positively excluded as the cause of a possible visceral pleural line (Fig. 54.5); external objects are easily recognizable if they have nonanatomical boundaries and extend beyond the thorax. Skin fold artifacts often manifest as a nonanatomical black line with fading margins, as opposed to the continuous white visceral edge of a pneumothorax, and vascular markings should also be traceable beyond a skin fold, but should terminate at the visceral edge of a genuine pneumothorax.
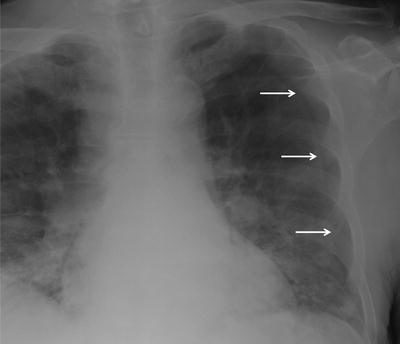

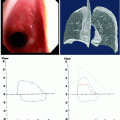
Fig. 54.1
Localized view of the junction lines on a CXR close up and associated CT. The anterior junction line (short arrows) is seen anterior to the mediastinum and does not pass up into the neck. The posterior junction line (long arrows) is seen posterior to the mediastinum and passes more cranial to the anterior junction lung
More recently, US has been shown to be of value in the detection of pneumothorax and is being increasingly used for their detection clinically, although patients with emphysema or bullous lung disease may be less easily assessed. It may also be difficult in trauma patients with surgical emphysema and may be false negative in patients with loculated pneumothoraces. MSCT is the gold standard in the detection and quantification of a pneumothorax, precisely reflecting pneumothorax size, permitting evaluation of underlying disease (such as emphysema, asthma, cystic fibrosis, and interstitial lung disease), and differentiating large bullae from a pneumothorax, although it may on occasion be difficult to interpret in some of these patients. It is also of value if associated surgical emphysema complicates radiographic interpretation. MSCT also identifies the presence of “tethered lung” adherent to chest wall and is the most valuable technique for guiding interventions in complicated cases and assessment of potentially malpositioned drains (Fig. 54.6).
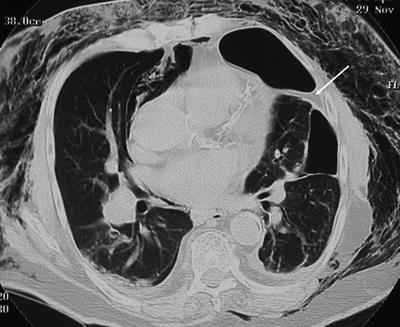

Fig. 54.6
Marked surgical emphysema prevented this left pneumothorax from being visible on the CXR. An arrow highlights the pleural adhesion that was also not visible
Although there are some suggested methods of deriving approximations of “percentage” volume from the CXR, these are not of additional clinical benefit to subjective (small or large) estimates or a single objective distance of maximum visceral pleural separation to describe interval change. Published guidelines have provided some simple rules; a >2 cm rim of air on an erect CXR is classified as a large pneumothorax and <2 cm as small; or an apex to cupola distance >3 cm as large and <3 cm as small.
Pleural Effusion
The normal volume of pleural fluid present in a healthy adult, 0.1–0.2 ml/kg, is not detectable by imaging, so when identified, is always secondary to either local or systemic disease. Pleural effusion may be either due to increased formation, decreased reabsorption, or a combination of both. The majority of clinically important pleural effusions result from cardiac failure, infection, and malignancy, although other diseases such as pulmonary embolic disease may only be suspected because of the presence of an effusion. The differentiation of transudates from exudates although broadly possible using imaging techniques is guided by fluid biochemical profile. The effusion appearance is highly dependent upon patient position, its underlying etiology, and the age or stage of its evolution. As described earlier, the CXR is usually the initial assessment and may be all that is required or performed in patients with known disease, for instance, cardiac failure. Further investigation and management is most commonly by US with CT and more complex imaging tests performed if needed following on from US. The detection of pleural effusions in supine and ICU films may be difficult with even moderate-sized effusions missed or misdiagnosed, and US plays a key role in these patients. It is often helpful to either tilt the patient into a slightly seated or erect position if possible, or to roll them away from the side being examined to help gain access more posteriorly with the ultrasound transducer. It is critical to take time and if necessary have help when performing an US on ICU to enable the examination to be performed adequately. Under these circumstances, US may help confirm the presence of an effusion and also aid in the decision on the need for thoracocentesis and/or drain insertion. US can confirm the presence of very small volumes of fluid and may help to differentiate between transudates and exudates. While the majority of pleural collections can be managed using radiography and US, MSCT is of particular benefit in pleural effusions of undiagnosed etiology, having the ability to evaluate pleural morphology, underlying lung parenchyma and identify other sites of disease and other etiologic causes. MRI is of limited additional benefit, although, similarly to US, it may exquisitely demonstrate pleural nodularity or thickening, and septations or loculations.
An erect PA image reliably demonstrates fluid volumes only when they are in excess of 200 ml, with the typical appearance being homogeneous lower zone opacity with a well-defined curvilinear lateral meniscus. A standard lateral film may demonstrate blunting of the posterior costophrenic sulcus when at least 25–50 ml of fluid is present. The most sensitive CXR technique for very small effusions is a lateral decubitus image, demonstrating as little as 5–10 ml fluid. In the supine position, fluid layers posteriorly and may be difficult to detect on a supine CXR. Positive signs on supine or semierect images include an indistinct hemidiaphragm or blunting of the costophrenic angle; an increase in radio-opacification of the hemithorax not obscuring the pulmonary vessels seen through it (Fig. 54.7); an “apical cap,” as the apical region may be the most dependent in the supine position; and fissural thickening. In the erect position, fluid preferentially accumulates between the inferior surface of the lung and hemidiaphragm, filling the costophrenic sulci, from posterior to anterior, resulting in an increase in radiographic density below the diaphragm on a PA and AP CXR before tracking up the convexity of the lung to blunt the costophrenic recess. Occasionally, the effusion accumulates only in a “subpulmonic” location and may be difficult to identify, although comparison with prior or sequential films may be helpful. The CXR appearances include the apparent elevation of the hemidiaphragm with a lateral shift of the apex, the cupola of the diaphragm, and a poorly defined costophrenic angle; absent bronchovascular markings; and a gastric air bubble >1.0 cm below the “presumed” hemidiaphragm. Pleural fluid extending into the fissures produces variable appearances often rapidly changing on sequential imaging, depending on the location and overall volume of fluid at the time of the CXR. Fluid in the oblique fissure usually causes a faint curvilinear opacity on frontal radiographs, which is commonly sharpest medially and fades away superiorly and laterally.
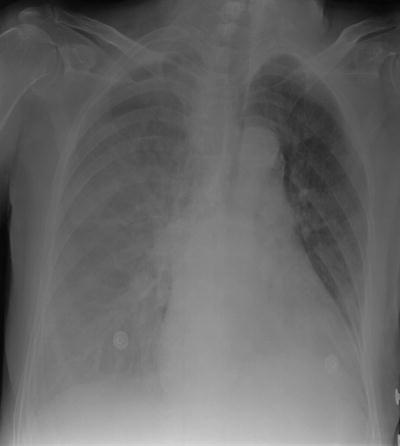

Fig. 54.7
A right-sided pleural effusion on a supine CXR, clearly demonstrated as an increase in radio-opacification of the hemithorax, but with the pulmonary vessels clearly seen through it
On US, fluid is of variable echogenicity. Transudates are most commonly anechoic but have recently been shown to occasionally demonstrate septations. Exudative effusions are of variable echogenicity. The US appearances may sometimes help to distinguish between etiologies, although anechoic effusions may very occasionally yield frank pus on aspiration. Fibrin strands and septae are commonly present in exudates, and these are easily seen on ultrasound and are usually associated with infected or malignant collections. They may be so profuse as to have a honeycomb appearance. Other signs of an effusion on US include a dynamic “flap” or “swirl” induced by respiratory or cardiac motion and caused by atelectatic and consolidated lung, or debris and septations. Malignant effusions more often have a positive swirling sign than benign disease, but this sign is not sensitive or specific enough for diagnostic purposes. Pleural thickening can be difficult to distinguish from pleural fluid, as both may be anechoic or only faintly hyperechoic. Color Doppler US may help to differentiate fluid from pleural thickening if there is doubt, as movement in fluid induced by respiratory or cardiac motion produces a positive “fluid color” sign as opposed to pleural thickening which provides little or no “fluid color” sign. Careful US examination for additional abnormalities seen on US such as pleural or diaphragmatic nodularity or a parenchymal lesion in adjacent atelectatic or consolidated lung may suggest a malignant etiology of the effusion. Sonographic assessment of the character and overall size of effusions has also been used to stratify patients with parapneumonic effusions into treatment with percutaneous pleural catheter drainage or medical thoracoscopy and to determine if or when intervention with video assisted thoracoscopy (VATS) is required. Recently, US has also been used in research studies to assess the efficacy of pleurodesis and absence of the “gliding sign” between parietal and visceral pleural surfaces during respiration strongly correlating with successful pleurodesis.
Pleural Effusion Etiology
Pleural effusions may occur in patients and be of a known etiology such as cardiac failure and malignancy, or occur in clinical circumstances that are suggestive of their etiology such as pneumonia and sepsis. They may also be the cause of presentation in patients without a known diagnosis such as pulmonary embolic disease or previously undiagnosed malignancy. The radiographic features are in part dependent upon their etiology and are here described according to etiology.
Parapneumonic Pleural Effusion and Empyema
Bacteria are by far the most common cause of pleural sepsis, with up to 40% of patients with pneumonia developing a pleural effusion, although only a minority of these progress to develop a complicated parapneumonic effusion or empyema. Viral parapneumonic effusions are much less common, as are effusions due to fungal infection, with the later usually occurring in association with other disease. Although the diagnosis ultimately relies upon fluid sampling and examination, imaging is an essential tool in management.
Complicated parapneumonic effusion and empyema may first be suspected on CXR in a patient with pneumonia who develops an effusion. No specific radiographic signs distinguish these from other effusions, although loculation, in the absence of a prior history of thoracic trauma, infection, or surgery, is suggestive of a “fibropurulent” or “organized” collection (Fig. 54.8); nondependent foci of gas within the fluid (from gas-forming organisms or following diagnostic tap or percutaneous catheter insertion) suggest the presence of septations, although these are infrequently seen on CXR and more commonly seen on US and MSCT.
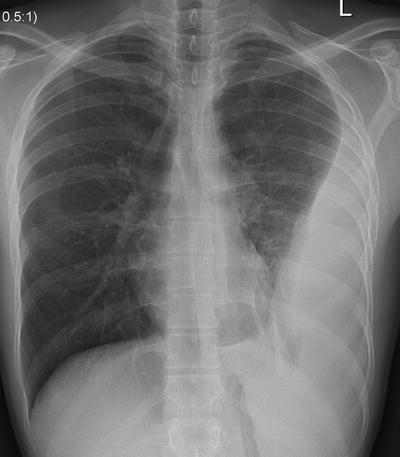

Fig. 54.8
A loculated left-sided complex parapneumonic effusion
A persistent hydropneumothorax following, incomplete drainage, with a thickened visceral pleura preventing reexpansion of the “trapped” lung may be identified on CXR and may necessitate surgical decortication.
US will confirm the presence of fluid and the signs of a complicated parapneumonic effusion such as echogenic fluid and septations (Fig. 54.9), and may demonstrate echogenic foci representing gas, particularly in multiseptated collections. The extent of the collection may not be appreciated by US, and although differentiation from consolidated lung is mostly straightforward, if the effusion fails to resolve with appropriate therapy, an underlying cause is unlikely to be detected. In these circumstances, MSCT may be of value. MSCT may be useful to demonstrate the full extent of an effusion, the degree of loculation, and potentially to guide pleural catheter insertion if patient anatomy or configuration of the collection makes US-guided intervention technically difficult. Although loculation is readily demonstrated on MSCT, septations are mostly not identified, although their presence may be inferred by nondependent gas collections. Several other CT features have been described in complicated parapneumonic effusions and empyema; the most frequently demonstrated finding is parietal pleural thickening and enhancement, which has a high sensitivity and specificity, compared with transudative effusions, and is present in over 90% of cases (Fig. 54.10). Extrapleural changes may also be seen in the subcostal tissues on MSCT, including increased thickness (>2 mm) and attenuation or stranding of the extrapleural fat and soft tissue, demonstrated in 70% of patients. Particularly when subtle, the changes may be best appreciated by comparison with the contralateral “normal” side.
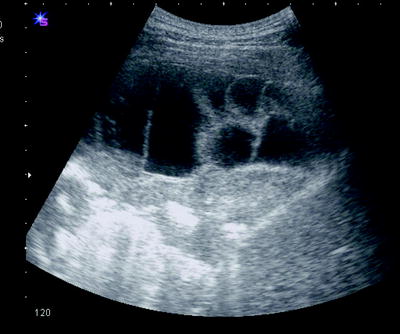
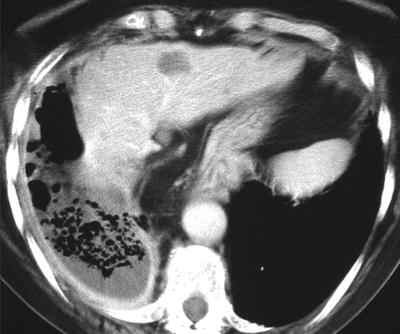

Fig. 54.9
A complex multiseptated pleural collection on US

Fig. 54.10
A right-sided empyema, demonstrating pleural enhancement, and multiple septations inferred by the presence of multiple pockets of gas. There is an increase in thickness and attenuation of the adjacent extrapleural fat and rib crowding
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree