Key Point
- ▪
Cardiac CT angiography is the test of choice to depict the entire course of coronary artery anomalies of termination. The conduit portions of the dilated segments are easier to identify than are the site(s) of drainage if those sites are multiple and small. Differential contrast attenuation may help reveal sites of drainage.
Coronary Artery Fistulae
A coronary artery fistula (CAF) is a solitary communication between a coronary artery and one of any of the following: cardiac chambers or arterial venous, coronary venous, or pulmonary arterial conduits—that is, a disorder of coronary artery termination. Coronary artery fistulae also may be of anomalous origin.
The incidence of CAFs in the population is unknown. The incidence within angiographic series is 0.1% to 0.2%, second in frequency of all coronary artery congenital abnormalities, after anomalous origin of the coronary arteries.
Coronary fistulae usually are congenital, but occasionally they may be posttraumatic or iatrogenic.
Most congenital coronary fistulae drain into adjacent cardiac chambers or vessels:
- □
The right heart atrium
- □
The right ventricle
- □
The pulmonary arteries
- □
The coronary sinus
- □
The superior vena cave: right atrial junction
- □
The hepatic veins
- □
The bronchial arteries
The incidence of multiple or large fistulae is approximately 0.05%, and the incidence of small fistulae is approximately 0.1% to 0.4% of angiographic series.
The right heart receives the drainage of 90% of congenital coronary artery fistulae; however, some congenital coronary artery fistulae may drain into the left heart.
Congenital fistulae may arise from:
- □
The left coronary artery: 50% to 60%, more commonly in the left anterior descending artery (LAD) than in the left circumflex artery (LCX)
- □
The right coronary artery (RCA): 30% to 40%
- □
Both coronary arteries: 2% to 5%
Although the RCA and LAD are the most commonly involved vessels, left circumflex coronary artery fistulae do occur, with drainage into sites such as the right atrium, the coronary sinus, and even the uncommon site of the left ventricle.
Multiple microfistulae from the coronary artery to the left ventricle have been described, arising off the distal portion of the coronary arteries. These have a female preponderance.
Typically, congenital coronary artery fistulae are conspicuously tortuous and have resulted in dilation (“flow-dependent dilation”) of the coronary arteries that feed them.
Most coronary artery fistula are asymptomatic. Potential complications are listed in the next sections of this chapter. Ischemia within the territory of the fistula may be detected scintigraphically or by evidence of prior infarction within the territory. In the absence of angiographically evident disease within the artery or fistula, the findings of ischemia or infarction likely implicate the fistula. Although the diagnosis of coronary steal is not easy to establish, it has been demonstrated in the setting of coronary fistula.
Spontaneous closure of congenital coronary fistulae has been reported.
Visualization of the distal portions of fistulae by CCT, especially if small, may be incomplete.
Etiologies of Coronary Artery Fistula
- □
Congenital
- □
Acquired
- •
Iatrogenic
- •
Aortocoronary bypass grafting
- •
Aortopulmonary artery
- •
Coronary artery-to-vein fistula
- •
- •
Congenital heart surgery repair
- •
Post- myotomy or post-myectomy for hypertrophic obstructive cardiomyopathy
- •
Endomyocardial biopsy
- •
Maze procedure
- •
- •
Aortic dissection
- •
Penetrating trauma
- •
Coronary artery dissection with rupture into an adjacent structure
- •
Coronary artery aneurysm with rupture into an adjacent structure
- •
Atherosclerosis
- •
Takayasu arteritis
- •
Neovascularization into a large left atrial thrombus
- •
Potential Complications
- □
Volume overload pathophysiology: congestive heart failure
- □
Infection: endovascular/endocarditis
- □
Coronary steal pathophysiology
- •
Ischemia
- •
Angina
- •
- □
Arrhythmia
- □
Syncope
- □
Aneurysm formation of the fistula
- □
Fistula rupture
- □
Fistula dissection
- □
Effusion
- □
Tamponade
- □
Hemorrhage/hematoma within the pericardial space
- □
Giant enlargement with compression of adjacent structures
- □
Coronary artery to left ventricular false aneurysm fistula
Patterns of Coronary Artery Fistulae
Coronary artery fistulae may occur in one or more permutations of:
- □
Source artery or arteries
- □
Drainage chamber(s) or venous structure(s)
- □
Absence or concurrence of congenital heart disease
- □
Absence or concurrence of acquired heart disease(s)
Hence, one fistula may drain into more than one low-pressure structure, and more than one fistula may drain into a single low-pressure structure, in the presence or absence of congenital or acquired heart disease.
Although the generalities of RCA and LAD source to right-sided or venous structures is the general rule, the range of permutations is vast and still incompletely known. A small sampling of infrequent but notably complex cases includes the following:
- □
Coronary artery to coronary sinus fistula (CACSF) may occur in association with congenital stenosis of the coronary sinus ostium and retrograde drainage via a persistent left superior vena cava.
- □
A case with four coronary to pulmonary artery fistulae also has been detailed.
- □
Coronary artery fistulae have been described in hypertrophic cardiomyopathy and mitral stenosis.
Potential CTA Findings of Coronary Artery Fistula
- □
Generally enlarged feeder vessel size
- □
Tortuosity of the fistula
- □
Aneurysm of the fistula
- □
Calcification of the fistula, generally in its dilated or aneurysmal portions
- □
Differential contrast/attenuation marking the site(s) of return of the fistula into chambers or vessels with lower attenuation
- □
Visualization of the surrounding chambers and hence the path of the fistula. The ability of cardiac CT to image the surrounding anatomy directly is the principal advantage when compared with conventional angiography, as is the overall robustness to manipulate a volumetric data set to solve the details of:
- •
Fistula source
- •
Fistula course
- •
Fistula site of termination
- •
Fistula complications such as aneurysm, calcification, thrombosis, hemorrhage
- •
Treatment
- □
Asymptomatic/small size: observation
- □
Asymptomatic/large size: intervention advocated by some
- □
Symptomatic/large size: consideration of intervention
Potential Interventions
- □
Endovascular
- •
Coiling (currently the most common catheter-based intervention)
- •
Otherwise: device closure, detachable balloons, plugs, various chemicals
- •
- □
Surgical
- •
Ligation
- •
Ligation with bypass
- •
Variants of CAF are illustrated as follows:
- □
CAF arising from the right coronary artery: Figures 10-1 and 10-2 and

Figure 10-1
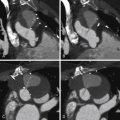
ECG-gated cardiac CT scan of a patient with a right coronary artery fistula. The feeder aspect of the fistula is the proximal right coronary artery, which is more dilated than the proximal left coronary artery. The proximal portion of the fistula is an aneurysm that gives rise to more than one exiting vessel. The aneurysmal portion is partially calcified. See Figure 10-2 and

Figure 10-2
Multiple volume-rendered images demonstrating a large right coronary artery to coronary sinus fistula. Note the marked enlargement of the coronary sinus. See Figure 10-1 and
- □
CAF arising from the left circumflex coronary artery: Figures 10-3 through 10-6 and

Figure 10-3
Volume-rendered images demonstrating a small coronary artery to coronary vein fistula extending from a second obtuse marginal branch to an inferior marginal coronary vein. See

Figure 10-4
Volume-rendered images demonstrate a markedly enlarged and tortuous circumflex artery fistulizing into a dilated coronary sinus. See Figure 10-5 and

Figure 10-5
Short-axis oblique images of a coronary angiogram with corresponding MR images demonstrating the enlarged circumflex artery, which mimicked a vascular retroatrial mass on echocardiography. See Figure 10-4 and

Figure 10-6
Two volume-rendered images demonstrate a small coronary artery to coronary vein fistula. The communication occurs between an obtuse marginal branch and marginal arterial branch and a small superolateral marginal vein, which is a tributary to the great cardiac vein. See
- □
CAF arising from the left main stem coronary artery: Figure 10-7 and

Figure 10-7
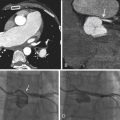
Left anterior oblique ( A ) and right anterior oblique ( B ) coronary angiography demonstrating a large fistula arising from the left coronary artery. C, Maximum intensity projection image revealing a fistula arising from the left main stem coronary artery and emptying into the distal coronary sinus. D, 3D reconstruction of the left main stem to coronary sinus fistula. See
- □
CAF draining into the pulmonary artery: Figures 10-8 through 10-14 and

Figure 10-8
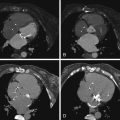
Two volume-rendered images from a cardiac CT study demonstrating a moderate-sized arteriovenous fistula from the left anterior descending artery to the main pulmonary artery. A small aneurysm is also noted.

Figure 10-9
A, A coronal reformation of a CT angiogram demonstrating a jet extending into the main pulmonary artery from a left anterior descending artery (LAD) to main pulmonary artery fistula. B, Cine gradient echo image from an MR study demonstrates a large coronary artery aneurysm with an associated fistulous connection from the LAD into the main pulmonary artery. Note the signal void at the level of the jet. See

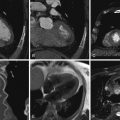
Figure 10-10
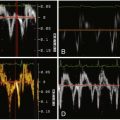
An 81-year-old woman presented with chest pain and an abnormal echocardiogram, raising suspicion for a possible patent ductus arteriosus. Multiple images from a cardiac MRI are shown. The upper two panels are gated fast spin-echo images showing multiple serpiginous signal voids in the region of the left main coronary artery as well a giant aneurysm with peripheral slow flow or thrombus, situated to the right of the main pulmonary artery. Cine gradient echo images confirm the large coronary aneurysm with an associated fistula into the main pulmonary artery. Note the plume of signal void in the main pulmonary artery ( D ) representing the fistula jet.

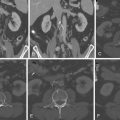
Figure 10-11
A, Three-dimensional volume-rendered reconstruction of the CT scan shows dilated branches of the right coronary artery (RCA) communicating with branches of the left anterior descending (LAD) artery, forming an aneurysmal Vieussens’ arterial ring (asterisk) . B, Coronary angiography, left anterior oblique cranial projection. The RCA was free of epicardial disease and showed two fistulae originating from the proximal third of the vessel and emptying into a vascular pouch before entering into the main pulmonary artery.
(Reprinted with permission from Hirzallah MI, Horlick E, Zelovitzky L. Coronary artery to main pulmonary artery fistulae via a Vieussens’ arterial ring. J Cardiovasc Comput Tomogr . 2010;4(5):339-341.)

Figure 10-12
Composite image demonstrates a left anterior descending artery (LAD) to main pulmonary artery fistula. An axial source image demonstrates the fistulous connection into the main pulmonary artery above the level of the pulmonic valve. Corresponding color Doppler echo images demonstrate the diastolic jet extending from the LAD into the main pulmonary artery. See
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree