Coronary Artery Disease: Perfusion with PET and SPECT
Philipp A. Kaufmann
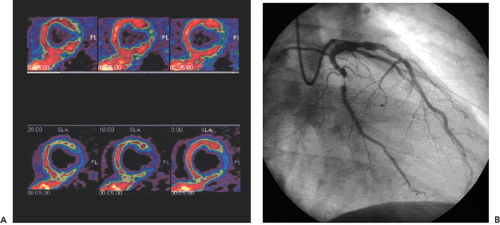
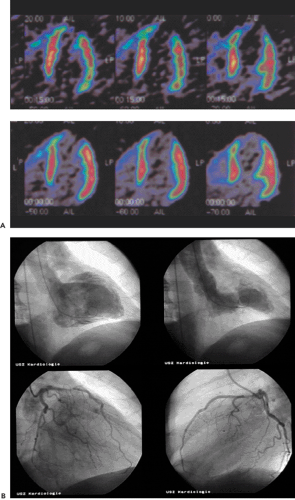
This chapter presents an overview of the impact of positron emission tomography (PET) and single-photon emission computed tomography (SPECT) myocardial perfusion imaging on the evaluation of patients with coronary artery disease (CAD). SPECT and PET are accurate tools for the noninvasive detection of CAD (see Fig. 29.1) in symptomatic and asymptomatic patients. PET is particularly useful in those patients with balanced three-vessel disease. PET also is often used in the assessment of physiologic stenosis severity to identify the culprit lesion as a target for revascularization strategy in patients with documented CAD. SPECT and PET permit assessment of the response to anti-ischemic and thrombolytic treatment as well as percutaneous transluminal angioplasty and surgical interventions designed to augment perfusion and improve function. Recently PET has been used for the detection of preclinical coronary endothelial dysfunction and the follow-up of progression or regression of CAD during risk factor modification.
Introduction
With the development of positron emission tomography (PET) technology, accuracy in diagnosis and prognosis has improved. In addition, PET provides an elegant tool for assessing metabolic processes in myocardial cells. In particular, it allows the distinction of viable myocardium from nonviable left ventricular segments after myocardial infarction. Accurate assessment of the extent of dysfunctional but viable myocardium (hibernating myocardium) is important in patients with large myocardial infarction and left ventricular dysfunction. With combined information from PET and coronary angiography, the best revascularization strategy can be found. For the detection of viable myocardium, PET offers positive and negative predictive values of 85% to 90% and 70% to 75%, respectively.
Coronary artery disease (CAD) detection and risk assessment continue to be mainstays of modern cardiology. They have gained in importance because of the broad range of therapeutic options now available to patients with CAD. These options range from cardiac surgery and catheter revascularization to modern pharmacologic treatment.
Basically, there are two possibilities for describing stenosis severity: anatomic determinants and physiologic determinants. The gold standard for the evaluation of CAD remains coronary angiography, which provides exact information on the location and anatomic severity of epicardial coronary artery stenoses. However, the interpretation of coronary angiographic images is affected by a considerable interobserver variability (1). This is underlined by the poor agreement of angiographic results in vivo with those after death (2,3). Furthermore, the physiologic significance of any lesion may be difficult to assess from angiographic images alone, and perfusion abnormalities may occur in the absence of arteriographically assessable stenoses. Angiographic evidence of stenosis severity is reported to show no or poor correlation
with clinical or physiologic parameters such as coronary flow reserve (CFR) and reactive hyperemia. Even the introduction of quantitative coronary angiography did not improve the predictive value of angiography. This explains the increasing importance of cardiac perfusion imaging in cardiovascular medicine. PET is the most advanced scintigraphic technique, allowing accurate qualitative and quantitative assessment of regional myocardial tracer distribution (4). PET has gained increasing clinical acceptance in cardiology.
with clinical or physiologic parameters such as coronary flow reserve (CFR) and reactive hyperemia. Even the introduction of quantitative coronary angiography did not improve the predictive value of angiography. This explains the increasing importance of cardiac perfusion imaging in cardiovascular medicine. PET is the most advanced scintigraphic technique, allowing accurate qualitative and quantitative assessment of regional myocardial tracer distribution (4). PET has gained increasing clinical acceptance in cardiology.
Two specific clinical applications of PET have been proposed for the evaluation of patients with CAD by the joint task force of the American College of Cardiology and the American Heart Association (5). The first is the noninvasive detection of CAD and estimation of the severity of the disease. This is dealt with in this chapter. The second clinical application of PET is the assessment of myocardial viability in patients with CAD and left ventricular dysfunction (see Chapter 30).
Evaluation of Coronary Artery Disease with PET
In a large number of studies, several primary clinical applications of PET perfusion imaging in CAD have been established:
Accurate noninvasive diagnosis of CAD in symptomatic or asymptomatic patients, including those with balanced three-vessel disease;
Assessment of physiologic stenosis severity for identification of the culprit lesion as a target for revascularization procedures in patients with documented CAD;
Assessment of response to anti-ischemic and thrombolytic treatment as well as percutaneous transluminal angioplasty and surgical interventions designed to augment perfusion and improve function;
Follow-up of progression or regression of CAD during risk factor modification; and
Assessment of microcirculatory dysfunction.
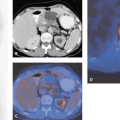
In daily clinical routine (5), diagnostic visual qualitative evaluation of nitrogen 13 [13N]ammonia or rubidium 82 (82Rb) PET perfusion images has proven feasible and accurate with a subjective scoring method of flow quantification rather than absolute flow quantification (6). The procedure is performed by using a PET perfusion agent at rest and during pharmacologic vasodilatation. The short half-lives of such agents permit rapid sequential examinations, such as rest dipyridamole studies, within a short time frame (1 to 2 hours). After intravenous administration, both [13N]ammonia and 82Rb distribute in proportion to regional blood flow. Images of the heart show deficits in regions where blood flow is relatively reduced and in zones of nonviable myocardium (e.g., previous myocardial infarction). The term “ischemia” is used loosely to denote impaired regional myocardial flow reserve during stimulation. Myocardial perfusion tracers indicate flow disparity between myocardial regions supplied by normal vessels and regions supplied by hemodynamically impaired vessels. Pharmacologically induced myocardial hyperemia is commonly used to cause such regional inhomogeneities in the perfusion pattern related to coronary stenoses, as illustrated in Figures 29.1 and 29.2. Physical exercise is less feasible in PET studies and has therefore only rarely been used.
Pharmacologic Stress
The common drugs used as substitutes for exercise stress testing are dipyridamole (Persantine), adenosine (Adenocard), and dobutamine (Dobutrex). Since the introduction of dipyridamole-induced coronary vasodilatation as an adjunct of thallium 201 (201Tl) myocardial perfusion imaging, pharmacologic interventions have become important in the noninvasive diagnosis of CAD. Excellent reproducibility of hyperemic flow response has been documented using PET for vasodilator (7) as well as for dobutamine (8).
Intravenous dipyridamole induces coronary vasodilatation indirectly by inhibiting cellular uptake of adenosine. This subsequently causes an increase in blood and tissue levels of adenosine, which is a potent direct coronary vasodilator and markedly increases coronary blood flow. Pharmacologically induced flow increase is of lesser magnitude through stenotic arteries, thus creating heterogeneous myocardial perfusion that can be visualized with a perfusion tracer. This mechanism may exist independent of myocardial ischemia, but in some patients true myocardial ischemia can occur with either dipyridamole or adenosine because of a coronary steal phenomenon. The accuracy of myocardial perfusion imaging with pharmacologic and physical stress has been found to be equal (9). Left bundle branch block may lead to false-positive septal defects with physical stress but not as much with pharmacologic stress (10). Before using dipyridamole or adenosine, patients must fast for 4 to 8 hours because of potential side effects of the stressors, such as nausea, vomiting, and hypotension. In addition, they must refrain from intake of caffeine-containing foods and beverages or aminophylline-containing drugs within 24 hours before the imaging study to prevent interference with the hyperemic effect.
High doses of dobutamine (20 to 40 μg/kg/min) elicit a secondary increase in myocardial blood flow (MBF) by increasing the three main determinants of myocardial oxygen demand: heart rate, systolic blood pressure, and myocardial contractility. Although the achieved flow increase (two- to threefold above baseline) is less than that elicited by the direct vasodilators mentioned earlier, it is sufficient to cause heterogeneous perfusion with radionuclide imaging. Overall, the accuracy is in the same range as that attainable with exercise, dipyridamole, or adenosine tests. Despite frequent side effects, high doses of dobutamine appear to be relatively safe.
Bicycle Exercise Stress
Adenosine or dipyridamole is commonly used as a hyperemic stimulus, although little is known about the interaction with drugs studied in pharmacologic interventions. Furthermore, the use of a pharmacologic stimulus may be of limited value in the assessment of the physiologic importance of any cardiovascular disease. Physical exercise is a physiologic stimulus that reflects the natural response of the coronary arteries to physical activities better than any isolated pharmacologic stimulation (11,12,13,14).
Nevertheless, only a few reports in the literature deal with the use of physical exercise in PET (15,16,17,18). Just recently we evaluated the feasibility and reproducibility of hyperemic MBF induced by supine bicycle exercise stress in the PET scanner and compared it with adenosine-induced hyperemic MBF. The mean CFR value for exercise stress was 1.9 ± 0.4, whereas the value for adenosine-induced flow reserve was 3.5 ± 1.0. Despite these differences, both stimuli revealed excellent reproducibility, as indicated by the mean difference of 5% for both stimuli (19). Thus we found that the assessment of CFR with oxygen 15 [15O]H2O and PET using bicycle exercise stress in the PET scanner is feasible and at least as reproducible as using adenosine stress. Our results seem to support the use of bicycle exercise stress in PET as a physiologic stimulus to induce hyperemic flow.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree