Abstract
Cortical neuronal cell development starts at about 7 weeks’ gestation from stem cells in the germinal matrix that line the ventricles. The stem cells proliferate and differentiate into glial cells and neurons. The glial cells migrate radially to the brain surface and create a scaffold for the neurons to follow to the outer cortex, where the neurons organize connections and commissures. Other neurons derived from the ganglionic eminence migrate tangentially to form inhibitory neurons and basal ganglia. Rapid development occurs at about 15 to 20 weeks’ gestation. By about 30 weeks’ gestation, neuron development is completed, resulting in the six cortical layers. Neurons are formed in larger numbers than needed, and more than half undergo apoptosis.
At the same time as the cortex is being formed, other brain structures develop, including the commissures (e.g., corpus callosum), cerebellum, and eyes. Interruption in normal neurodevelopment from any cause can result in abnormalities of any of these structures, and the final clinical manifestations often reflect the timing rather than the nature of the insult.
Migration and development are controlled by complicated gene-protein interactions. Genes controlling neural development often also control the development of other body organs such as muscles and bones. Mutations or disturbances to genes involved in neurogenesis can result in abnormal somatic development. For example, mutation in the FGRF3 gene associated with thanatophoric dysplasia results in both skeletal abnormality and cerebral pachygyria.
Keywords
abnormal migration, abnormal proliferation, hemimegalencephaly, heterotopia, lissencephaly, macrocephaly, microcephaly, polymicrogyria, schizencephaly
Introduction
Cortical neuronal cell development starts at about 7 weeks’ gestation from stem cells in the germinal matrix that line the ventricles. The stem cells proliferate and differentiate into glial cells and neurons. The glial cells migrate radially to the brain surface and create a scaffold for the neurons to follow to the outer cortex, where the neurons organize connections and commissures. Other neurons derived from the ganglionic eminence migrate tangentially to form inhibitory neurons and basal ganglia. Rapid development occurs at about 15 to 20 weeks’ gestation. By about 30 weeks’ gestation, neuron development is completed, resulting in the six cortical layers. Neurons are formed in larger numbers than needed, and more than half undergo apoptosis.
At the same time as the cortex is being formed, other brain structures develop, including the commissures (e.g., corpus callosum), cerebellum, and eyes. Interruption in normal neurodevelopment from any cause can result in abnormalities of any of these structures, and the final clinical manifestations often reflect the timing rather than the nature of the insult.
Migration and development are controlled by complicated gene-protein interactions. Genes controlling neural development often also control the development of other body organs such as muscles and bones. Mutations or disturbances to genes involved in neurogenesis can result in abnormal somatic development. For example, mutation in the FGRF3 gene associated with thanatophoric dysplasia results in both skeletal abnormality and cerebral pachygyria.
Disease
Definition
Disturbances of cortical development are collectively called malformations of cortical development . They are very diverse, and there is no single or complete classification. A commonly used classification by Barkovich et al. classifies malformations of cortical development according to time of insult and genetic abnormality ( Table 36.1 ).
|
Prevalence and Epidemiology
Malformations of cortical development are uncommon. However, about 25% of children and young adults with intractable epilepsy are found to have malformations of cortical development.
Etiology and Pathophysiology
The basic abnormality is a disturbance in normal proliferation, migration, and organization of neurons from any cause. The age when the insult occurs and disturbs a specific development phase can be more important than the specific nature of the insult in determining the type of malformations that occur. The etiology of malformations is varied. About half result from as yet unknown intrinsic fetal causes; the remainder include single gene disorders, and inborn errors of metabolism (peroxisomal disorders, organic acidopathies, mitochondrial disorders). Extrinsic (acquired) insults include hypoxia, maternal diseases (diabetes, phenylketonuria), and maternal exposures (teratogens).
Manifestations of Disease
Clinical Presentation
There is a large range of presentation from normal function to severe developmental delay or death. Epilepsy is common. Many individuals have additional problems related to associated anomalies and syndromes.
Imaging Technique and Findings
The brain undergoes significant morphologic changes throughout gestation. It is important to be aware of normal appearances at each age.
Ultrasound.
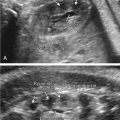
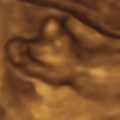
Ultrasound (US) is the mainstay of prenatal fetal central nervous system (CNS) evaluation. Additional information can be obtained using Doppler and three-dimensional (3D) evaluation. Cortical sulci and the insula follow a regular pattern of maturation that can be assessed by 18 weeks’ gestation. The medial hemispheric sulci are more readily assessed with US ( Fig. 36.1 and Table 36.2 ).

| Fissure or Sulcus | US | MRI | ||
|---|---|---|---|---|
| First Seen | Always Seen | First Seen | Always Seen | |
| Parietooccipital fissure | 18.5 | 20.5 | 18–19 | 23–23 |
| Calcarine fissure | 18.5 | 21.9 | 18–19 | 22–23 |
| Cingulate fissure | 23.2 | 24.3 | 24–25 | 28–29 |
| Central sulcus | 26–27 | 26–27 | ||
| Convexity sulci | 23.2 | 27.9 | 26–27 | 28–29 |
Magnetic Resonance Imaging.
Magnetic resonance imaging (MRI) is the second line of investigation and allows more complete anatomic delineation ( Fig. 36.2 ). MRI can be more effective in evaluation of cortical surfaces and gray and white matter differentiation, and can detect hypoxic and hemorrhagic changes. Increasingly sophisticated MRI sequences are being developed to evaluate metabolic and microstructural changes in the cortex. Currently MRI effectiveness in the diagnosis of cortical malformations remains limited before 24 weeks.


Specific Malformations of Cortical Development
Microcephaly
Definition
Microcephaly is defined, in the pediatric population, as occipitofrontal head circumference less than 2 standard deviations (SD) below the mean for age and gender. Some authors believe less than 3 SD is more appropriate, and likely to be of clinical significance in the diagnosis of fetal microcephaly. Microcephaly is a descriptive term; it does not refer to a specific pathologic condition. The intention is to identify infants with small heads who are at risk for mental retardation.
Prevalence and Epidemiology
Prevalence is estimated at 1 : 10,000 to 1.6 : 10,000 births. Microcephaly is commonly seen in association with other conditions. The incidence is likely higher if spontaneous abortions, stillbirths, and neonatal deaths are included. Depending on etiology, failure of head growth may manifest in early or late pregnancy, but in the vast majority of cases it only becomes evident during the first year of life.
Etiology and Pathophysiology
The etiology is very heterogeneous ( Table 36.3 ). The change in head size reflects failure of brain growth. Smaller heads are more likely to be associated with neurodevelopmental deficiency. Online Mendelian Inheritance in Man (OMIM) lists 680 conditions associated with microcephaly ( www.ncbi.nlm.nih.gov/sites/omim ). There are two main categories: primary (congenital) and secondary (acquired) (see Table 36.3 ).
| PRIMARY (CONGENITAL) |
|
| SECONDARY (ACQUIRED OR ENVIRONMENTAL) |
|
Manifestations of Disease
Clinical Presentation
The head appears small; in the fetus the occipitofrontal circumference is less than 3 SD below the mean for gestational age. Microcephaly may manifest at any age ranging from early second trimester to after delivery. Often there is a suspicious pregnancy or family history. In one group of 33 affected children, findings were as follows: 48% had distinct cerebral malformations, 18% had findings of cytomegalovirus (CMV), 24% had nonspecific brain abnormalities, and 10% had no overt brain abnormality. Of the 90% of children with identifiable brain abnormalities, 93% were neurodevelopmentally abnormal. Other infections such Zika virus (ZIKV) can cause cerebral disturbances and microcephaly.
Imaging Technique and Findings
Ultrasound.
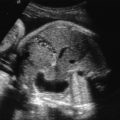
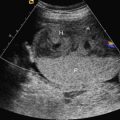
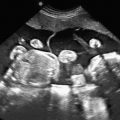
Head circumference is small for age (less than 3 SD below the mean for gestational age). As a rule of thumb, generally the biparietal diameter is about 10 mm smaller than expected, and age estimated from biparietal diameter is about 4 weeks too small. In some cases, there is an abnormal head-to-abdomen ratio or head-to-femur ratio, or both. The forehead is short and slopes backward ( Fig. 36.3 ), and there is an increased subarachnoid space. Brain structures are poorly visible (owing to decreasing US windows, as calvarial collapse narrows sutures and fontanelles), requiring scanning in atypical planes to see intracranial structures (see Fig. 36.3 ). Overall, the brain usually appears abnormal (e.g., structural abnormalities, abnormal sulcation [often simplified], holoprosencephaly, calcifications with infections). There may be other fetal findings related to possibly associated syndromes. In the absence of associated anomalies, the diagnosis of microcephaly is rarely suspected before delivery.

Magnetic Resonance Imaging.
MRI findings are similar to those of US and include (see Fig. 36.3 ) brain smaller than expected for skull (wide subarachnoid spaces); abnormal gyration pattern, often simplified; and possibly signs of infection (calcifications, stranding in ventricles). However, MRI allows more detailed evaluation of sulci and gyri for abnormality and may show areas of hemorrhage or ischemia that are not evident on US. In addition, noncerebral findings may suggest an etiology and associated syndromes.
Small head
Short and sloping forehead
Brain structures hard to see with US and usually dysmorphic
Other anomalies
- •
Head circumference less than 3 SD below mean for gestational age
- •
If head circumference is less than 2 SD below mean for gestational age, repeat the examination in 3 weeks
- •
Head growth failure may appear late
- •
Multiple etiologies
- •
Multidisciplinary investigations and counseling useful
Macrocephaly and Hemimegalencephaly
Definition
Macrocephaly refers to head enlargement from any cause, including abnormalities of the scalp, cranial bones, and intracranial structures. It is a descriptive term and is defined as occipitofrontal circumference greater than 2 SD above the mean for gestational age and sex. Some authors use 98th centile or greater than 3 SD above the mean for gestational age. The term megalencephaly describes a brain that is large. Macrocephaly may be due to megalencephaly (true enlargement of brain parenchyma) or many other conditions that cause an enlarged head. Usually obvious conditions that can cause head enlargement, such as hydrocephaly and brain tumors, are not included. Hemimegalencephaly is a malformation of cortical development that manifests as a hamartomatous enlargement of one hemisphere.
Prevalence and Epidemiology
Prevalence is not quoted, but by definition would include about 2% of the population; in these cases, most of the macrocephalic individuals will be normal. Macrocephaly is a finding associated with various conditions ( Table 36.4 ). OMIM lists 218 conditions associated with macrocephaly and 27 associated with megalencephaly ( http://www-ncbi-nlm-nih-gov.easyaccess2.lib.cuhk.edu.hk/omim ).