2 Craniocerebral Trauma
Closed and Open Head Injuries
Closed Head Injuries
A head injury is classified as “closed” if it leaves the dura intact. Unlike gunshot injuries, for example, traumatic head injuries caused by a force acting over a large area (motor vehicle accidents, falls) do not penetrate the dura. Because the dura is firmly adherent to the inner table of the skull, however, it may be lacerated by a skull fracture (Fig. 1.9 b).
Open Head Injuries
An open head injury is one in which the dura has been breached (Fig. 1.13 b). These injuries are generally caused by a violent force acting on a small area, as in a gunshot injury or an impact from a sharp-pointed object. Open head injuries include frontobasal fractures and temporal bone fractures with cerebrospinal fluid (CSF) leakage. Immediate surgical treatment is a priority in open head injuries, to reduce the ever-present risk of infection.
Skull Fractures
Frequency: a common computed tomography (CT) finding in patients with head injury.
Suggestive morphologic findings: discontinuity in the bone; diploë not separated from the fracture line by cortical bone; other linear discontinuities.
Procedure: if plain skull films demonstrate a fracture, obtain thin-slice cranial CT scans to exclude intracranial hemorrhage.
Other studies: plain skull films are good for demonstrating calvarial fractures, but it is essential to detect or exclude an intracranial mass lesion.
Checklist for scan interpretation:
 Fracture depressed (by more than the width of the calvarium)? Burst fracture (vertex) or bending fracture?
Fracture depressed (by more than the width of the calvarium)? Burst fracture (vertex) or bending fracture?
 Intracranial displacement of fragments?
Intracranial displacement of fragments?
 Intracranial air signifying dural injury?
Intracranial air signifying dural injury?
 Accompanying hemorrhage or contusion?
Accompanying hemorrhage or contusion?
 Pathogenesis
Pathogenesis
Skull fractures should not be considered in isolation, but must be viewed in connection with lesions of the brain parenchyma and other intracranial structures (blood vessels, cranial nerves). A skull fracture is commonly associated with cranial nerve lesions, vascular injuries, and dural injuries that create portals for the entry of air and microorganisms and for CSF leakage. At the same time, the inability to visualize a fracture does not exclude extensive cerebral contusions or intracranial hematomas. Generally, it is not the fracture itself that is of primary interest in head injuries (at least not a calvarial fracture) but the space-occupying or disruptive intracranial lesions that may accompany the fracture.
 Frequency
Frequency
• Nearly one-third of head-injured patients do not have a skull fracture, including cases with a fatal outcome.
• When a skull fracture is detected, the likelihood of brain injury is increased 30-fold.
 Clinical Manifestations
Clinical Manifestations
The clinical manifestations of skull fractures are as varied and diverse as the potential sites of occurrence.
• A petrous bone fracture may be manifested by bleeding from the ear or a collection of blood behind the tympanic membrane.
• Frontobasal fractures are often associated with unilateral or bilateral periocular hematomas (“monocle” or “eyeglass” hematomas).
• Fractures of the sphenoid wing may cause blindness due to rupture or infarction of the optic nerve.
• If the fracture involves the sella, avulsion of the pituitary stalk can lead to hormonal deficits.
• Fractures of the cribriform plate often result in anosmia.
• The cranial nerves most commonly involved by basal skull fractures are the trochlear nerve, trigeminal nerve (first and second divisions), facial nerve, and vestibulocochlear nerve.
 CT Morphology
CT Morphology
Fractures are clearly demonstrated by CT when the fracture line is perpendicular to the scanning plane (Fig. 2.1). Fractures that run along the plane of the scan may be missed.
 Differential Diagnosis
Differential Diagnosis
Cranial sutures should not be mistaken for fracture lines. Differentiation is aided by noting that with a suture, the diploë is separated from the gap by cortical bone. In a fracture, however, the diploë borders directly on the fracture gap. Comparison with the opposite side also helps to identify sutures. Vascular channels generally have a visible sclerotic margin.
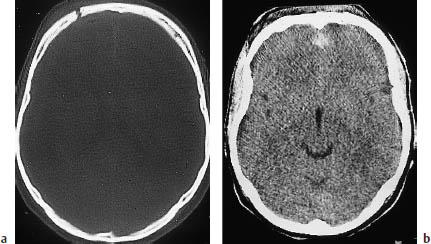
Fig. 2.1 Spectrum of CT findings in open head injury. Many such injuries result from a blow to the face or forehead, creating a coup-contrecoup mechanism with marked occipital lesions and less severe frontal lesions. The scans above show a discontinuity in the right anterior calvarium, consistent with a frontal skull fracture (a, bone window). Scanning with a soft-tissue window, just below the scan level in a, shows blood in the frontal interhemispheric fissure (b), with no other significant signs of injury. Intracranial air bordering the fracture line in panel a signifies an open skull fracture.
The main role of CT in follow-up is to check for CSF leakage in patients who have sustained an open head injury. Moreover, follow-up is generally advised in patients with initially mild-appearing parenchymal injuries such as contusions and in patients with small intracranial hemorrhages, particularly if clinical deterioration cannot be inferred from the patient’s neurologic status because of intoxication or other causes.
Cerebral Contusion
Frequency: a common CT finding in head-injured patients.
Suggestive morphologic findings: acute hypodense lesions, usually located in the frontal or temporal region. Hyperdense hemorrhage appears after a variable delay.
Procedure: schedule CT follow-ups postoperatively and as dictated by intracranial pressure (herniation, hemorrhage after decompression).
Other studies: magnetic resonance imaging (MRI) is more sensitive than CT in the subacute stage (especially with diffuse axonal injury and other shearing injuries; see below).
Checklist for scan interpretation:
 Location and extent of contusions? Hemorrhagic contusions?
Location and extent of contusions? Hemorrhagic contusions?
 Associated injuries (calvarial fracture, basal skull fracture, petrous bone fracture, subdural or epidural hematoma, traumatic subarachnoid hemorrhage)?
Associated injuries (calvarial fracture, basal skull fracture, petrous bone fracture, subdural or epidural hematoma, traumatic subarachnoid hemorrhage)?
 Pathogenesis
Pathogenesis
Contusions are caused by acceleration or deceleration trauma to the head (Fig. 2.2). The lesions are characterized morphologically by edema of the brain tissue, with a variable hemorrhagic component.
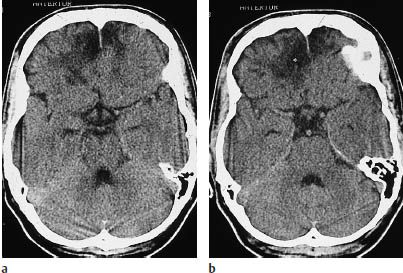
Fig. 2.2a, b Cerebral contusion. Contusions appear on CT as patchy hypodensities. A hemorrhagic component may be absent, as in the bifrontal contusions shown here.
The location of cerebral contusions is governed by the following principles:
• Relative differences in the acceleration and deceleration of the calvarium and brain cause forces to act over a broad area of brain tissue according to the coup-contrecoup principle: the brain tissue is first injured at the site of impact (the calvarium is accelerated toward the underlying brain) and then on the opposite side of the skull (the moving brain strikes the inner surface of the decelerating calvarium). The contrecoup lesions may be more severe than the coup lesions at the site of impact.
• Most contusions occur in the frontal or temporal brain (Fig. 2.3), presumably partially due to the rough-edged bony structures that occur in those areas. Occipital contusions are less common owing to the smooth inner surface of the occipital calvarium.
• Shearing injuries are also common in head-injured patients. They are caused by the shear strain that develops at the gray-white junction due to the different acceleration properties of the gray and white matter. The lesions consist of axonal injuries and the rupture of very small vessels. The corpus callosum and subcortical white matter are most commonly affected. Hemorrhage within contusions can be identified on CT scans. Although MRI can demonstrate increased T2-weighted signal intensity with much greater sensitivity, practical problems have prevented the use of this modality, at least in the early stages.
 Frequency
Frequency
Examination of trauma patients is a field for CT, and cerebral contusions are consistently detected in patients with relatively severe head trauma.
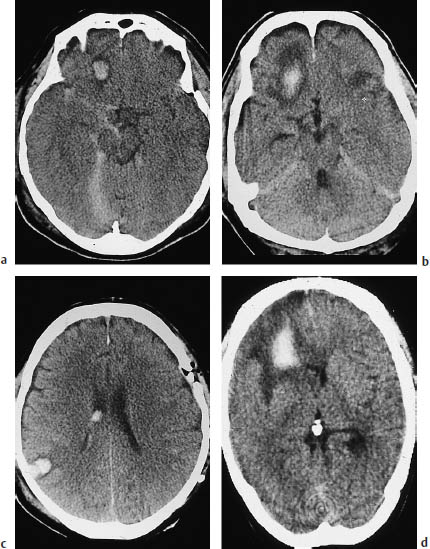
Fig. 2.3 Spectrum of CT findings in contusion. These scans illustrate the typical features of hemorrhagic contusions in different patients. a A small hemorrhage rimmed by hypodense edema is visible in the right frontal lobe. There is associated traumatic subarachnoid bleeding in the right basal cisterns. b A large hemorrhagic contusion with prominent perifocal edema. c Coup and contrecoup lesions. A fracture is evident in the left frontoparietal calvarium, and a hemorrhagic contusion is visible on the opposite side in the area of the right parieto-occipital junction. Intraventricular blood is also present on the right side. d The extensive hypodensity almost filling the entire right frontal lobe is a hemorrhagic area at the center of a contusion. A small ring artifact is visible in front of the internal occipital protuberance.
 Clinical Manifestations
Clinical Manifestations
 CT Morphology
CT Morphology
Contusions appear on CT scans as hypodense areas that are located within the white matter but may also occasionally involve the cortex. As hemorrhage increases, the lesion may show varying degrees of increased density or layering.
 Differential Diagnosis
Differential Diagnosis
Contusional hemorrhage, which may have a delayed onset, is not always distinguishable from hypertensive intracerebral hemorrhage. The clinical features at the time of CT examination are not helpful for differentiating the pathogenic mechanisms. This is a particular problem if there is uncertainty as to whether trauma was the cause or result of the intracerebral hemorrhage (e.g., rupturing an aneurysm on the steering wheel of an automobile).
 Evolution of Lesions
Evolution of Lesions
With passage of time, cerebral contusions lead to tissue breakdown, with the formation of parenchymal defects of variable size. This occurs even in nonhemorrhagic contusions. In some cases, however, even severe contusions may undergo almost complete resolution without causing significant cavitation.
 Follow-Up
Follow-Up
As noted at the start of this chapter, CT has an important role in the follow-up of contusions, especially since an enlarging contusion with increasing mass effect frequently leads to clinical deterioration.
Epidural Hematoma
Frequency: less common than subdural hematoma, but frequent in cases with extensive skull fractures in patients with head injuries.
Suggestive morphologic findings: hyperdense, biconvex mass usually bordering the calvarium in the temporoparietal area. The adherence of the dura to the cranial sutures prevents the mass from crossing the sutures.
Procedure: immediate surgical decompression.
Other studies: MRI is indicated in very rare cases (subacute epidural hematoma, hematoma near the vertex).
Checklist for scan interpretation:
 Report CT findings without delay.
Report CT findings without delay.
 Location (temporal lobe!) and maximum diameter?
Location (temporal lobe!) and maximum diameter?
 Measured degree of midline shift?
Measured degree of midline shift?
 Associated injuries (subgaleal hematoma, calvarial fracture)?
Associated injuries (subgaleal hematoma, calvarial fracture)?
 Hypodense inclusions signifying active hemorrhage?
Hypodense inclusions signifying active hemorrhage?
 Pathogenesis
Pathogenesis
Bleeding into the epidural space usually has an arterial origin, i.e., damage to the middle meningeal artery or one of its branches. A subgaleal hematoma and calvarial fracture are often found at the site of the impact. The classic causal mechanism of epidural hematoma is the rupture of a meningeal arterial branch caused by a calvarial fracture, as these vessels generally are firmly adherent to the bone. Epidural hematoma displaces the dura away from the inner table of the skull.
 Frequency
Frequency
Epidural hematomas are not uncommon in patients with head injuries.
The intracranial collection forms quickly after the trauma (with arterial bleeding), distinguishing it from the more insidious development of a subdural hematoma. Unless the epidural hematoma is evacuated without delay, the resulting mass effect can lead to brain herniation and death.
 CT Morphology
CT Morphology
CT demonstrates a hyperdense, biconvex, peripheral mass in direct contact with the calvarium (Fig. 2.4) and showing a smooth interface with the cerebral convexity.
The smooth medial border of an epidural hematoma is defined by the underlying dura. The hematoma may still appear isodense shortly after the trauma, but its density increases as clotting progresses and the plasma components are reabsorbed. Because hematomas of arterial origin develop rapidly, different stages of coagulation are not observed, and consequently the hematoma usually presents a homogeneous density. The mass effect from the hematoma almost always causes a shift of midline structures, which may be extensive. The temporoparietal location of most epidural hematomas may cause herniation of the medial temporal lobe, with displacement of the parahippocampal gyrus and uncus into the tentorial incisure.
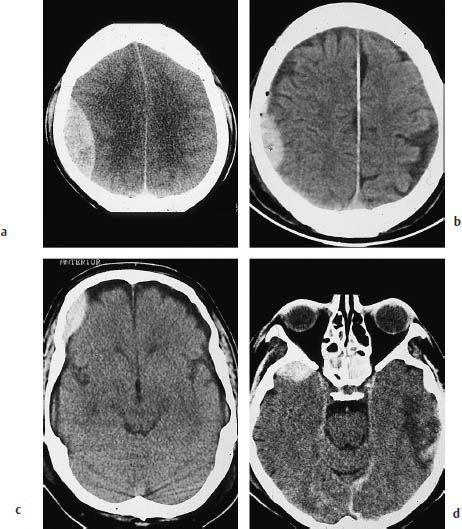
Fig. 2.4 Epidural hemorrhage. Epidural hematoma typically appears as a lentiform collection with a convex medial border. a A large parietal epidural hematoma, associated with a definite midline shift. b A smaller epidural hematoma. The tiny air bubbles near the hematoma often signify open head trauma with dural injury. c An epidural hematoma located anterior to the coronal suture. Unlike subdural hematomas, epidural hematomas do not cross cranial sutures. d A small epidural hematoma overlying the right temporal pole. A hematoma at this location is usually visible only in the temporal lobe projection. Contusional changes are visible on the left side.
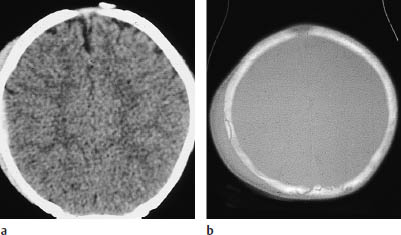
The fixation of the dura to the inner table of the skull is variable. The dura is firmly adherent to the calvarium in very young patients (Fig. 2.5) and in the elderly, and so epidural hematomas are less common in these age groups.
The dura is very firmly attached at the cranial sutures. If the classification of a hematoma is unclear, it is assumed to be subdural if it crosses a suture. With older hematomas, the relationship of the mass to the dura may be the only criterion available for epidural/subdural differentiation. With an epidural hematoma, the dura appears as a thin linear density between the hematoma and cerebral surface. Often this is evident on plain CT scans, and it is almost always visible after contrast administration.
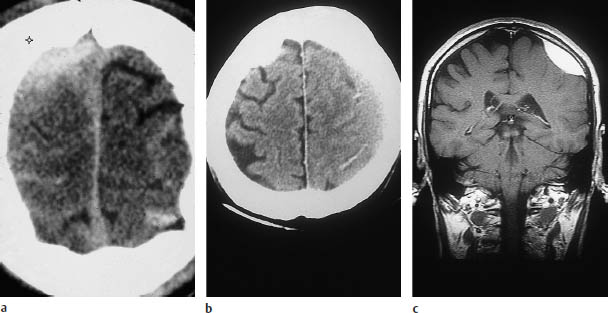
Fig. 2.6 A special case of an epidural hematoma located near the vertex. As noted in the text on p.  , a blow to the vertex has special significance. Injuries to the sagittal sinus are common, and small epidural hematomas may be missed.
, a blow to the vertex has special significance. Injuries to the sagittal sinus are common, and small epidural hematomas may be missed.
a This scan shows a hematoma in the right superior frontoparietal area that was classified as epidural by coronal magnetic resonance imaging (MRI). In sideto-side comparison, note the sulcal effacement and peripheral hemorrhages on the right side. Such injuries are often caused by a falling object striking the head (construction site injury, etc.).
b Same patient as in a.
c Coronal MRI demonstrates the now-subacute hematoma more clearly, owing to its high signal intensity. A high epidural hematoma located near the midline often requires angiographic evaluation of the dural
Infratentorial epidural hematomas are rare and take a fulminating course. Care should be taken not to overlook epidural hematomas located near the vertex (Fig. 2.6).
Coronal reconstructions may be necessary to demonstrate these hematomas and evaluate the adjacent bone. Their relationship to the superior sagittal sinus may require contrast angiography.
 Differential Diagnosis
Differential Diagnosis
Differentiation is mainly required from subdural hematoma. The differentiating criteria are described above, although there are cases in which the usual criteria are difficult to apply, especially when findings are minimal. However, the imaging findings and an accurate localization of the mass to the epidural or subdural space are less important than the degree of mass effect and the severity and acuteness of the clinical findings in deciding whether to proceed with surgical evacuation.
Traumatic Subarachnoid Hemorrhage
Frequency: often coexists with other traumatic brain hemorrhages.
Suggestive morphologic findings: hyperdense blood on the cerebral surface, in the interhemispheric fissure (differential diagnosis: subdural hematoma), or on the tentorium.
Procedure: thin-slice CT examination with a bone window is appropriate in some cases. If the distribution of blood suggests a ruptured berry aneurysm, the lesion can be demonstrated by intravenous contrast administration.
Other studies: lumbar puncture is more sensitive for detecting subarachnoid hemorrhage. MRI has practically no role in acute diagnosis, due to its relative insensitivity to unclotted blood.
Checklist for scan interpretation:
 Location and extent?
Location and extent?
 Follow-up: malabsorptive hydrocephalus?
Follow-up: malabsorptive hydrocephalus?
 Blood in the ventricular system (usually nontraumatic; obstructive hydrocephalus)?
Blood in the ventricular system (usually nontraumatic; obstructive hydrocephalus)?
 Pathogenesis
Pathogenesis
Traumatic subarachnoid hemorrhage may occur in isolation after a head injury or may be associated with other traumatic lesions (epidural/subdural/intracerebral hematomas, contusions, fractures).
 Frequency
Frequency
Subarachnoid hemorrhage is frequently detected in severely head-injured patients and may be accompanied by bleeding into other compartments.
 Clinical Manifestations
Clinical Manifestations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




