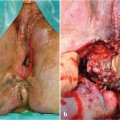
Fig. 12.1
American Gastroenterological Association classification of perianal disease
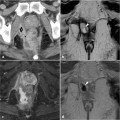
At transanal ultrasound, fistulous tracts may be identified as hypoechoic, round to oval structures that lie in any location within the anal wall. They can be internally hyperechoic (increased or bright echoes) if they contain gas. According to the Parks classification, intersphincteric fistulas are characterized by a primary tract that courses in the intersphincteric space without penetrating the external sphincter (Fig. 12.2). Transsphincteric fistulas traverse the external sphincter and pass into the ischianal fossa, below the level of the puborectalis muscle (Fig. 12.2). Suprasphincteric fistulas course within the intersphincteric plane superior to the puborectalis before penetrating the levator musculature to course within the ischiorectal and ischioanal fossae (Fig. 12.2). Extrasphincteric fistulas course within the ischioanal fossa and penetrate the levator musculature without traversing either the internal or the external sphincter, opening directly into the rectum (Fig. 12.2).
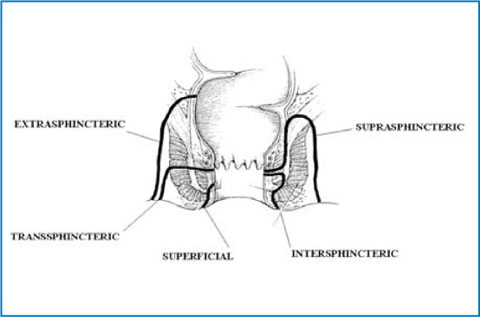

Fig. 12.2
The Parks classification of perianal fistulas
Besides the classification of fistulous tracts, it is important to report the site of the internal opening. This is very effectively identified by transanal ultrasound because the opening lies right at the probe surface. The opening may be detected as a gap or disruption of the mucosal integrity and is usually reported by describing the site (anterior, posterior, left, lateral), often using a clockface description (e.g., 12 o’clock anterior, 6 o’clock posterior, 3 o’clock left, 9 o’clock right lateral).
Along with the description of the primary fistula tract and the site of its internal opening, any branching, secondary tracts or areas of extension must be identified and reported. It is well known that missed extensions are the most common cause of fistula recurrence after treatment [8]. Extensions may include blind-ending tracts and abscesses. These can be identified as an ane- choic (without internal echoes) or hypoechoic mass (with internal echoes due to cellular debris or gas). When an abscess extends to either side of the internal opening it is described as a “horseshoe” abscess (Fig. 12.3). However, as transanal ultrasound is limited to the transversal plane, it is somewhat difficult to determine whether this extension is infralevator or supralevator. To overcome this difficulty and to improve the overall accuracy of transanal ultrasound, three-dimensional (3D) and contrast-enhanced ultrasound have been introduced. 3D transanal ultrasound is a volumetric acquisition of multiple parallel two-dimensional ultrasound images that are synthesized into a 3D data set, which may be manipulated digitally as an imaging volume. In contrast-enhanced transanal ultrasound a contrast agent, usually a 3% solution of hydrogen peroxide at a dose 2.5–5 ml, is injected before or during the examination into the external opening of a draining fistula, either directly using a syringe or by using a 16-G intravenous catheter connected by a short tube [9, 10]. With this technique, perianal fistulas may be easily classified and the identification of extensions is both more accurate and simpler. However, it should be taken into account that this technique is feasible only in active draining fistulas. In patients with intermittent drainage, it may be difficult to introduce the catheter into the external orifice if it is temporarily closed. In this case, the patient should be examined without contrast agent and the contrast examination rescheduled. In addition, the passage of hydrogen peroxide into the lumen of the fistula may be slow or incomplete in fibrotic and inactive fistulas and may require an appropriate length of time to completely delineate the fistula and its extensions.
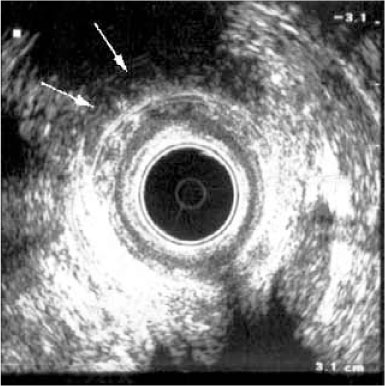

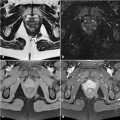
Fig. 12.3
Transanal ultrasound of the upper anal canal level in a male patient, showing a hypoechoic horseshoe extension well appreciable on the left side (arrows)
The accuracy of transanal ultrasound in evaluating CD perianal fistulas was assessed in several studies. A study from the Mayo Clinic [9] compared transanal ultrasound, magnetic resonance imaging (MRI), and surgical exploration under anesthesia (EUA), showing that more than one technique is required to fully describe and classify fistulas. In that study, 34 patients with suspected perianal fistulas were prospectively enrolled and their fistulas classified according to the Parks criteria. The accuracy of all three modalities was ≥85%: transanal ultrasound 91%, MRI 87%, and EUA 91%. However, accuracy was 100% when any two tests were combined.
The examination with contrast agent, especially when combined with 3D volumetric acquisition, has also proven to be superior to conventional ultrasound in demonstrating and delineating fistula tracts and in identifying internal fistula openings. A study investigating 41 patients with CD and perianal fistulas showed that a 3D contrast-enhanced examination can reveal a greater number of surgically difficult-to-treat fistulas (in up to 78% of patients, in the study) compared to conventional transanal ultrasound, suggesting that the technique is mandatory before surgery [10].
12.2.2 Assessment of Activity
Quantitative assessment of the activity of perianal fistulas in patients with CD may be of clinical relevance in disease management. Various clinical and instrumental methods are used for this purpose, such as fistula drainage assessment (FDA), the perianal disease activity index (PDAI), and MRI [12–16]. Transanal ultrasound also has been proposed for the assessment of perianal fistula activity. In general, active fistulas are visualized as intense hypoechoic tracts sometimes containing focal areas of hyperechogenicity consistent with gas bubbles, whereas inactive fistulas are visualized as less hypoechoic tracts lacking any hyperechoic foci [12, 14]. However, these ultrasonographic criteria in assessing fistula activity are limited by the visual ability to judge grayscale echogenicity, which also may be influenced by the brightness and shadowing of surrounding structures. To overcome this limitation, Caprioli et al. suggested the quantitative computerized analysis of digitalized images of fistulas. The authors assessed fistula tract activity using both a subjective index and a computerized method. They found that active fistulas have a hypoechoic appearance that can be quantified by calculating the mean gray-scale tone [17]. According to the subjective index, the active fistula is described as a hypoechoic tract occasionally containing focal hyperechoic areas representing gas bubbles within, the inactive fistula is defined by the presence of less hypoechoic or isoechoic bands without any hyperechoic foci. The computerized method consists of the statistical image-analysis of gray-scale tones with in a region of interest (ROI), as drawn using a computer mouse, on fistula tracts. This determination is assigned a number ranging from 0 (dark) to 255 (white) (Fig. 12.4). The authors showed that the agreement between clinical examination and transanal ultrasound was fair (k-value = 0.266-0.294), whereas that with computer-assisted analysis of anal ultrasound was good (k-value = 0.608-0.670) and quite similar to that between clinical examination and MRI (k-value = 0.739). These results were confirmed in another study by the same group, which showed that the gray-scale tone cut-off discriminating active from inactive fistula tracts is 117–118. It is worth noting that in the latter study transanal ultrasound was found to be complementary to other clinical scales and useful to assess perianal disease activity in CD [18]. Unfortunately, this method, although simple, is relatively time-consuming, unless specific software is integrated in the ultrasonographic device.
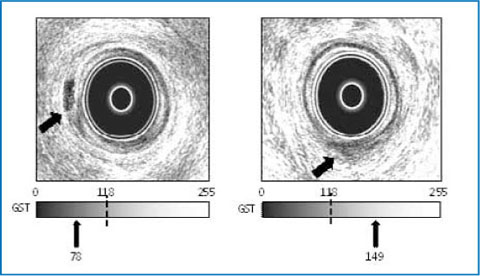

Fig. 12.4
Endosonogram of an active (left) and an inactive (right) perianal fistula, based on a gray-scale tone cut-off value discriminating active from inactive fistula. The overall gray-scale tone of the regions of interest taken at different levels of the fistula tract indicates the activity of the fistula
12.2.3 Follow-Up and Prognostic Findings
Over the last decade, anti-tumor necrosis factor-α (anti-TNF-α) antibodies (infliximab and adalimumab) have considerably improved the medical treatment of fistulizing CD. This class of drugs has proven to be an effective medical option for fistulizing CD, with a rate for the short-term cessation of fistula drainage > 65% [4]. However, both treatments also account for a high rate of abscess formation (up to 15%) due to premature closure of the cutaneous openings of the fistula tract [19




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree