Chapter Outline
Nomenclature for CT and MR Enterography Findings
Additional Imaging Considerations
Clinical Considerations
Crohn’s disease is an idiopathic inflammatory disease that can affect any part of the gastrointestinal (GI) tract from the mouth to the anus. Patients with this disease have a genetic predilection to an abnormal immunologic response to environmental factors, including food, and gut flora leading to a chronic inflammatory response. The small bowel is the major site of involvement. With the exception of malignant neoplasms, Crohn’s disease can be the most devastating disease to involve the GI tract. Its prevalence has increased, mostly in younger age groups, with a peak age between 15 and 25 years. It has a worldwide distribution but is most common in northern Europe, North America, and Japan. Four studies have shown a bimodal distribution, with the first peak in the second or third decade of life and the second, smaller peak in the sixth or seventh decade. However, four other studies have shown a unimodal distribution, peaking in the second or third decade, and decreasing thereafter. The colonic predominance in older adults has led some to speculate that the second peak incidence in this cohort is caused by ischemia or diverticulitis. Both genders are equally affected, although some studies have shown a slight female predominance. A familial tendency has frequently been described.
The small bowel is commonly involved by Crohn’s disease, with the terminal ileum the most common location. In a review combining multiple population-based studies on the natural history of adult Crohn’s disease, approximately one third of patients had ileitis, one third had colitis, and one third had ileocolitis. Isolated perianal disease occurs in 2% to 3%, but perianal or rectal fistulae are common in colonic or rectal disease (10% to 37%). Upper GI tract involvement is being recognized with increasing frequency but almost invariably occurs with small bowel or colonic disease. The gastric and duodenal manifestations of Crohn’s disease are discussed in Chapter 30 , and the colonic features of this disease are discussed in Chapter 58 . Although the focus of this chapter is on Crohn’s disease of the small bowel, there are differences in presentation from colonic disease. Patients with colonic Crohn’s disease are more likely to present with blood loss, perianal disease, toxic colitis, and extraintestinal complications. Crohn’s disease in the small bowel has a slightly better prognosis, although there are more likely to be complications such as abscesses, fistulae, and obstruction. Abdominal pain, mild diarrhea, weight loss, and pyrexia are common clinical findings. Other patients may present with a right lower quadrant mass, representing the diseased ileum or cecum. Delays of 3 to 4 years have been reported between the onset of often subtle symptoms and the diagnosis of Crohn’s disease in the small bowel.
Many factors contribute to the development of diarrhea in patients with Crohn’s disease. An inflamed bowel mucosa causes increased secretion of fluid and electrolytes. Extensive terminal ileal disease or resection impairs bile salt reabsorption, leading to the malabsorption of fat and fat-soluble vitamins. Bacterial overgrowth secondary to strictures, fistulae to the colon, adhesions, aneurysmal dilation, or bypassed loops can also cause diarrhea. The combination of decreased food absorption and intermittent obstruction may lead to significant weight loss in these patients.
Classification and Therapy
Over the years, attempts have been made to classify Crohn’s disease anatomically, clinically, and operatively. This has evolved from the International Working Party in 1991 to the Vienna classification in 1998, and finally to the Montreal revision of the Vienna classification in 2005.
The Montreal revision classifies the disease into age of diagnosis, location, and disease behavior. Disease behavior is classified into nonstricturing, nonpenetrating (uncomplicated, active inflammatory alone), stricturing, and penetrating, with the addition of perianal disease as a modifier. It is the behavior subcategory that is of interest in regard to the radiographic manifestations. As a result of these morphologic subtypes, radiologists and even gastroenterologists have concluded that they are separate entities. The problem with this discrete approach is that the complex relationship between active inflammatory and stricturing disease and stricturing and penetrating disease has been forgotten or avoided. Crohn’s disease is a progressive process, starting with acute inflammation. It may be stopped or retarded with therapy; there may be mucosal healing. However, when it progresses, it often progresses to the fibrostenotic phase, with stricture formation. Active inflammation commonly coexists in the presence of stricture formation. There is also strong pathologic and clinical evidence that fistulae form in the face of fibrostenosis. Two pathologic series have shown that fistula formation without stricturing disease is uncommon to rare (4% to 7% of the time). Thus, if there is no stricture, penetrating disease (sinus tracts, fistulae, abscess, or free perforation) will be very unlikely. Because of the potential progression of disease from acute inflammation to fibrostenosis and then fistulization, it is important for the radiologist to think more broadly than using the Montreal and Vienna classifications, especially with computed tomography (CT) and magnetic resonance (MR) enterography. We are thus beginning to use the following terms— active inflammatory disease, mixed active inflammatory and fibrostenotic disease, fibrostenotic disease, and inactive or quiescent disease with the addition of penetrating disease (generally in the presence of mixed disease) based on radiographic findings (see later, “ Nomenclature for Computed Tomography and Magnetic Resonance Enterography Findings ”).
Medications useful for the active inflammatory subtype of Crohn’s disease include 5-aminosalicylate-based drugs, corticosteroid-based drugs, and antibiotics (e.g., metronidazole, ciprofloxacin). Second-line medications are immunosuppressive agents such as azathioprine, 6-mercaptopurine, and methotrexate. Targeted monoclonal antibodies such as infliximab, which targets human tumor necrosis factor-β, and natalizumab, which targets β 4 integrin, a leukocyte adhesion molecule, have become available. These drugs are very expensive and have complications. This arsenal of medications has prompted the need for noninvasive, reproducible, and objective measurements of Crohn’s disease activity, especially the distinction between active inflammatory disease from mixed fibrostenotic and active inflammatory disease with or without penetrating disease. Some centers of excellence would argue that penetrating disease can only be treated with surgery rather than medical therapy.
The fistulizing-perforating type requires antibiotics initially to treat the active infection. Monoclonal antibodies have proven effective in patients with perianal disease and enterocutaneous fistulae. However, internal fistulae (enteroenteric, enterocolic, enterovesicular, and complex internal fistulae) are much more difficult to treat medically. Percutaneous abscess drainage is a suitable means of treating Crohn’s disease–related abscesses. The fibrostenotic subtype of Crohn’s disease may need strictureplasty or bowel resection because small bowel obstruction is the major clinical manifestation of this type. The quiescent or reparative type of Crohn’s disease may require maintenance medication.
Diagnostic Tools
There are many examinations available for diagnosing, staging, and following Crohn’s disease of the small bowel. These include ileocolonoscopy with direct inspection and biopsy of the terminal ileum, push enteroscopy to examine the proximal and mid small bowel, video capsule endoscopy, conventional small bowel follow-through, conventional enteroclysis, CT with (CTE) or without enterography or enteroclysis, MR enterography (MRE) or enteroclysis, ultrasonography, and lately positron emission tomography (PET), PET/CT, and PET/MRI. These studies are used to do the following:
- •
Demonstrate the early changes of Crohn’s disease.
- •
Depict the full extent of involvement and the possible presence of skip lesions if surgery is contemplated.
- •
Determine the cause of any clinical deterioration in previously stable patients with Crohn’s disease.
- •
Distinguish between spasm (active inflammatory disease), mixed active and fibrostenotic disease, and fibrostenotic disease (stricture formation); increasingly used to dictate medical versus surgical therapy, especially with MR enterography.
- •
Investigate postoperative complications of Crohn’s disease.
- •
Definitively rule out the presence of Crohn’s disease in the small bowel, especially in patients with an indeterminate colitis.
- •
Ileocolonoscopy and Video Capsule Endoscopy
Ileocolonoscopy ( Figs. 41-1 and 41-2 ) allows the accurate diagnosis of Crohn’s disease of the colon and terminal ileum. It provides assessment of disease activity and consequences of inflammation visually and with guided biopsy, including strictures, mass lesions, bleeding, and the development of dysplasia or malignancy. In general, this examination is considered the gold standard for diagnosis.





Video capsule endoscopy is a new means of directly inspecting the entire length of the small bowel. It uses an ingested sealed capsule that measures 27 mm in length and 11 mm in width. The camera within the capsule, which also contains a light source, takes two photographs at eight times magnification every second and runs on electricity supplied by onboard batteries. During the typical small bowel examination, an average of about 55,000 photographs are taken. The capsule is propelled through the GI tract by peristalsis. The images are transmitted in radiofrequency signals to and stored on a recorder device placed in a belt worn by the patient throughout the day of the procedure. The capsule is disposable and is not recovered after the test. At the end of the study, the recorder device is retrieved and the images obtained are downloaded onto a computer. The computer generates a video that is then reviewed for relevant pathology.
Early studies have shown video capsule endoscopy (see Fig. 41-2B ) to be the most sensitive means of diagnosing nonstricturing Crohn’s disease. However, the presence of a potentially obstructing stricture is a major contraindication for the use of capsule. Furthermore, many if not most of the studies that have shown the high sensitivity have not had corroborating reference standards. The studies that compared capsule with CT enterography-enteroclysis and/or MR enterography-enteroclysis have only shown that capsule is superior for disease more proximal to the terminal ileum, with no significant difference between the imaging studies and capsule in the terminal ileum. The only reference standard used to demonstrate the increased diagnostic efficacy of capsule over imaging has been the capsule findings. Even a recent four-center Danish trial, which showed that capsule is superior to CTE or MRE, only had the terminal ileum as a reference standard (5 of 93 had surgery); also, in this trial, a capsule examination was only performed after the enterography showed no stricture. In this study, the superiority of capsule was for proximal disease, not the terminal ileum.
Capsule is well known to be highly sensitive but relatively low in specificity. In a comparison of standard small bowel examination, ileocolonoscopy, CTE, and capsule performed in Crohn’s patients at the Mayo Clinic, the specificity of capsule was only 53% (sensitivity, 83%). In this study, the sensitivity and specificity of CTE and ileocolonoscopy were 82% and 89% and 74% and 100%, respectively.
Barium Contrast Examinations
In this new millennium, the barium–fluoroscopic barium examination of the small bowel for Crohn’s disease has been largely supplanted by CTE and MRE. However, there are patients who have medical conditions that preclude the use of CTE or MRE with contrast enhancement (e.g., severe chronic kidney disease) who have known or suspected Crohn’s disease. These patients must be evaluated with a barium examination using fluoroscopy.
There are two methods of examining the small bowel with barium using fluoroscopy—the standard small bowel series and enteroclysis. For accuracy, both require meticulous fluoroscopic technique using patient rotation and graded compression with a lead glove, inflatable paddle, or wooden spoon. Enteroclysis is available only in certain centers and requires intubation of the proximal small bowel via the nose or mouth. This is uncomfortable for most patients, and conscious sedation is used in some centers to improve patient tolerance.
As a general rule, the key radiographic finding in Crohn’s disease is asymmetry. Radiologists usually think of asymmetry as skip disease in the z direction (see Figs. 41-1A, Fig. 41-2C, and Fig. 41-6 ). However, the asymmetry of Crohn’s is also in the xy direction (see Fig 41-2C ). Crohn’s disease always affects the mesenteric more than the antimesenteric border. The normal undulating fold pattern on the mesenteric border will become ulcerated, effaced, flattened, and foreshortened, whereas there is relative sparing of the antimesenteric border, giving rise to the pseudodiverticulosis sometimes associated with the disease (see Fig. 41-5 ). This classic feature is better portrayed with barium examinations, but can be seen with CT or MR enterography.
Marshak’s ten principles of Crohn’s disease are worth remembering because they assist the radiologist in detecting and characterizing disease. Initially presented in 1973, these principles have generally held very true. The principles most helpful for the radiologist include principle 3 (the string sign is the most pathognomonic barium manifestation and does not represent fibrosis but spasm secondary to ulceration), principle 8 (following surgery, recurrence is common), and principle 9 (look for recurrence at anastomotic and strictureplasty sites).
Early Active Radiographic Inflammatory Disease
The pathologic and radiologic findings correlate well in early Crohn’s disease. The earliest histologic changes consist of hyperplasia of lymphoid tissue (see Fig. 41-1A ) and obstructive lymphedema in the submucosa. Because the submucosa extends into the core of mucosal folds, the folds may thicken at this stage in a smooth, symmetric manner. However, in general, symmetric fold thickening in Crohn’s disease is an uncommon finding, and is identified only early in the disease. When the disease advances, the folds become much more asymmetrically thickened, giving a macronodular polygonal appearance; the macronodules vary in size (at this point, the main differential is lymphoma; see later).
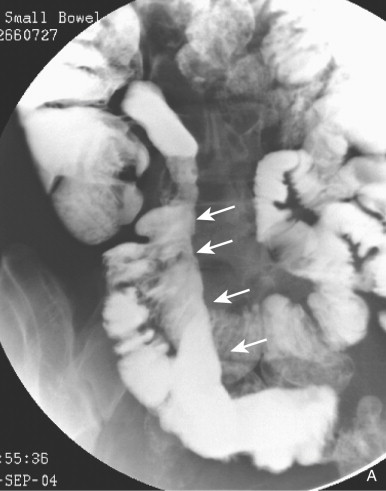
Hyperplasia of lymphoid follicles ( see Fig. 41-1 ) in the lamina propria can be associated with shallow, 1- to 3-mm mucosal erosions surrounded by a small halo of edema, also known as aphthoid ulcers (see Fig. 41-2 ).
Intermediate Radiographic Active Inflammatory Disease
As the disease gradually extends transmurally, further changes take place in the mucosa and submucosa. Progressive submucosal edema may cause the base of the folds to widen until some folds are partially or completely obliterated or effaced, especially on the mesenteric side. This process is similar to the thumbprinting that occurs in patients with bowel ischemia. In patients with ischemia, however, the fold abnormalities change over a period of days to weeks, whereas in patients with Crohn’s disease, the fold abnormalities persist and are usually associated with changes of more advanced Crohn’s disease elsewhere in the small bowel. The mucosal inflammatory infiltrate tends to vary focally in intensity. This infiltrate, together with patchy submucosal fibrosis, leads to a distortion and interruption of folds. The fold pattern in this stage can be described as macronodular (>1 cm), with the nodules asymmetric in size, location, and appearance.
Some aphthoid ulcers may enlarge and deepen, producing a stellate or rose thorn appearance. Other aphthoid ulcers may fuse with adjacent ulcers, producing crescentic or linear shapes. A typical finding is that of a long linear ulcer on the mesenteric border, which may be accentuated by a parallel radiolucency caused by fusion of thickened, transaxially oriented folds. The mesenteric border ulcer is associated with thickening, sclerosis, and retraction of the adjacent mesentery and of the straightened mesenteric border of the involved bowel. At the same time, the unaffected, or relatively unaffected, redundant antimesenteric border becomes pleated, scalloped, or sacculated, giving a pseudodiverticular-like pattern (distinct from the pseudodiverticular pattern present in scleroderma, in which the outpouchings are much closer together because of the foreshortening of the bowel).
An inflammatory cellular infiltrate with focally pronounced edema and granulation tissue can give rise to localized mucosal elevations or inflammatory polyps. These lesions are common in the colon but infrequent in the small bowel. Inflammatory pseudopolyps usually occur in small numbers in an area of mucosa that is denuded of folds. Occasionally, a bowel segment contains many inflammatory pseudopolyps (≤1 cm in diameter), separated from one another by curving lines of barium occupying the crevices between the elevations. When seen in profile, the polyps appear as notches demarcated by protrusions of barium. The diameter of these bowel segments is not reduced. This is called the nodular pattern of Crohn’s disease to underscore its essential difference from the ulceronodular, or cobblestone, pattern of advanced, fissuring, ulcer-related Crohn’s disease ( Fig. 41-3 ).

Advanced Radiographic Active Inflammatory Disease
In advanced Crohn’s disease, the process has extended transmurally to the serosa and beyond. Deep linear clefts of ulceration, or fissures, are typical of this stage of the disease ( Fig. 41-4 ). Islands of surviving mucosa surrounded by extensive ulceration give the appearance of elevations above the ulcerated background. These islands of mucosa are therefore known as pseudopolyps. The deep fissures characteristic of this advanced stage may be manifested by a combination of axial and transaxial fissuring, which separates the pseudopolyps from one another. This finding is always associated with luminal narrowing; it is referred to as the ulceronodular or cobblestone pattern of advanced Crohn’s disease (see Fig. 41-3 ). This pattern should not be confused with the nodular pattern of less advanced Crohn’s disease.


Changes associated with linear mesenteric ulceration advance in a caudad direction as the disease progresses. Antimesenteric redundancy of the opposite bowel wall gradually disappears as it is incorporated in the transaxial extension of ulcerated Crohn’s disease ( Fig. 41-5 ). The bowel wall is now thickened by a combination of fibrosis and inflammatory infiltrate. Another feature of transmural disease is the so-called fat wrapping that occurs as the hypertrophied subperitoneum is tethered toward the bowel wall by mesenteric perivascular fibrosis.

Quiescent or Inactive Disease
Very little has been written about the barium findings of quiescent disease. Presumably, the mucosa is normal in these patients. Thus, the fold pattern should be normal.
Fibrostenotic Disease
The strictures of small bowel Crohn’s disease ( Figs. 41-6 and 41-7 ) are caused by collagen deposition, predominantly in the submucosa. It is important to differentiate fibrostenotic strictures from the luminal narrowing that can result from spasm. The classic string sign of Crohn’s is primarily caused by spasm in segments of active inflammatory disease. Fluoroscopically, the lumen, coated with barium, appears initially narrowed, with no or only mild upstream dilation. Then, when a peristaltic wave propels the barium through the lumen, the lumen expands to a near-normal caliber. A fibrostenotic predominant stricture will not change its caliber with peristalsis, and the upstream, nonaffected lumen will be dilated, regardless of the state of peristalsis. It should be noted that in many if not most cases of the string sign, the lumen of the affected bowel never fully distends, and there is often some degree of upstream ballooning or dilation during peristalsis. This suggests that more than spasm is occurring in the affected segment (Crohn’s is a progressive process; at sites of active disease, there is often active proliferation of smooth muscle and collagen that restricts compliance).



These strictures are an important cause of small bowel obstruction, often necessitating surgery. Strictures or stricture-like findings are reported in 21% of patients with small bowel Crohn’s disease. MDCT, magnetic resonance imaging (MRI), CT enterography, CT enteroclysis, and MR enteroclysis are valuable techniques for differentiating fibrotic strictures from lumen narrowing by spasm or active ulcerated stenotic disease. It is important to note that active inflammation is present in many, if not most, fibrostenotic strictures (see later). Obstruction by a fibrous stricture may require surgery; whenever possible, it should consist of strictureplasty to avoid bowel resection. Some patients with Crohn’s disease may develop high-grade small bowel obstruction; this complication is reliably documented by CT and MR.
Other patients with Crohn’s disease may have multiple strictures, representing advanced skip lesions. Stasis related to strictures can be associated with bacterial overgrowth. This is especially true in patients who develop aneurysmal dilation of the small bowel (see Fig. 41-7 ), usually between two strictures. Strictures are generally well shown by barium study.
Resection of bowel in patients with Crohn’s disease is followed by a high frequency (85%) of disease recurrence. Recurrent inflammation has been detected within 8 days of surgery, provided that exposure to the fecal stream had been reestablished. Multiple resections have made Crohn’s disease of the small bowel a major cause of the short bowel syndrome.
Fistulizing or Penetrating Disease
Fistulizing or penetrating disease is manifest by sinus tracts, fistulae, abscess, and uncommonly free perforation. Abscesses develop in some 20% of patients with Crohn’s disease, and most can be treated with percutaneous drainage. Inflammatory lesions may remain closely related to a diseased segment of bowel or may extend beyond the bowel, occasionally into the psoas muscle. In some cases, barium may enter an abscess cavity, a collection of tracts, or multiple small spaces within an inflammatory mass. However, CT, ultrasonography, and MRI are preferred diagnostic methods for evaluating abscesses in patients with Crohn’s disease.
Fistulae are abnormal communications between two epithelial surfaces or an epithelial surface and the skin, which occur in 6% to 33% of patients with Crohn’s disease. Fistulae occur in the presence of, and likely as a result of, stricture formation (fistulae are only rarely found in isolation, only 4% to 7% of the time; Fig. 41-8 ). Ileocecal, ileosigmoid, and enteroenteric fistulae are the most common and are often multiple. An enterocolic fistula may lead to bacterial overgrowth and is one of the causes of malabsorption associated with Crohn’s disease. Enterocutaneous fistulae can be well shown by barium studies, CT, and MRI.
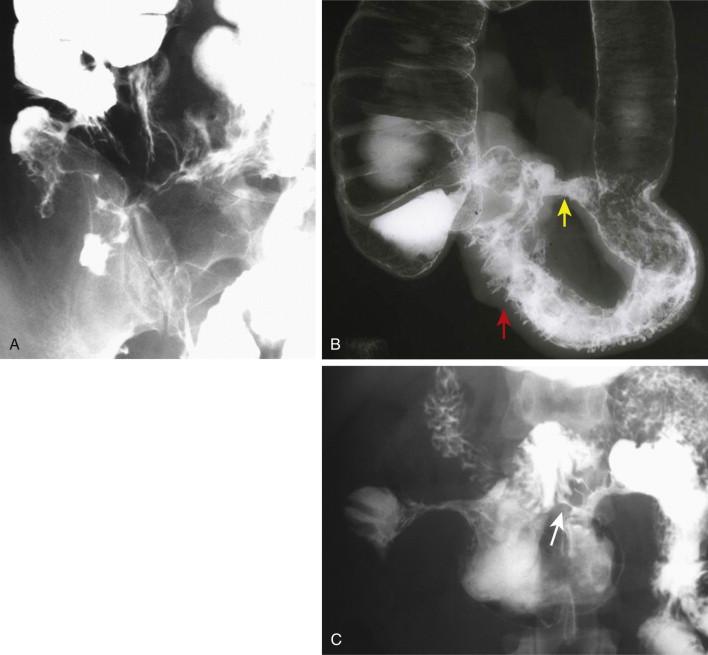
Because the attachment of the transverse mesocolon crosses the mid-descending duodenum, Crohn’s-related fistulae between the transverse colon and duodenum are not unusual. Recurrent Crohn’s disease in the neoterminal ileum after ileotransverse colonic anastomosis may be associated with such a fistula. Ileosigmoid fistulae are encountered more often. These may serve a useful purpose by bypassing strictures in the ileocecal area. In most cases, the entry site of these fistulae into the sigmoid colon shows only nonspecific inflammatory changes. If surgery is contemplated, it is usually adequate to resect the diseased ileum and stricture, leaving the sigmoid colon intact.
Disease Activity as Assessed by Barium
In the 1970s, the National Cooperative Crohn’s Disease Study attempted to determine whether barium examinations could accurately assess response to therapy and whether barium findings correlated with clinical response, as judged by the Crohn’s Disease Activity Index (CDAI). Patients were divided into those with active, symptomatic disease (CDAI > 150; n = 295) and patients with disease that had been active, but was now quiescent (CDAI < 150; n = 274). The investigators concluded that the findings on barium examination did not correlate with clinical symptoms or response. In general, there was little improvement of barium findings during the study; only patients treated for longer than 6 months with prednisone showed a statistically significant improvement in findings.
Ultrasound
Ultrasound has been increasingly used to assess Crohn’s disease, especially in the pediatric population. The examination requires meticulous, systematic scanning of all parts of the bowel, using graded compression and a high-frequency transducer after the administration of oral contrast. Recently, investigations have shown the added benefit of contrast-enhanced ultrasound in assessing Crohn’s disease, especially in differentiating fibrosis from active inflammation. Mural thickening ( Fig. 41-9 ) is the most common abnormality seen in patients with Crohn’s disease of the small bowel. It is typically concentric, and the mural echogenicity depends on the degree of inflammatory infiltration and fibrosis. In early acute disease, mural stratification is retained. With long-standing disease, a target or pseudokidney appearance may be identified. In patients with burned out, long-standing disease, fat deposition in the submucosa may be present.




Actively inflamed gut appears rigid and fixed, with decreased or absent peristalsis. Color Doppler imaging typically shows hyperemia. Findings on spectral Doppler analysis include increased superior mesenteric and/or inferior mesenteric artery blood flow, increased pulsatility index, decreased resistive index, and increased portal vein velocity. Increased systolic and diastolic flow through the superior mesenteric artery may also be seen, attesting to disease activity. Creeping fat of the mesentery manifests as a uniform echogenic halo around the mesenteric border of the encased gut. As with barium study, MDCT, and MRI, this abnormal fat causes separation of bowel loops.
Prominent lymph nodes are seen in most patients with the active inflammatory phase of Crohn’s disease. These perienteric lymph nodes, which are located in the subperitoneal space of the small bowel mesentery, present as focal hypoechoic masses that are spherical and have lost their normal echogenic streak emanating from the nodal hilum. These nodes are typically hyperemic, moderate in size, and tender. Abnormal lymph nodes are not commonly seen in quiescent Crohn’s disease.
Crohn’s disease strictures show mural thickening of the gut, with the luminal surfaces of the involved segments in apposition ( Fig. 41-10 ). The lumen may appear as a narrow, linear, echogenic central region within the thickened segment of small bowel. Dilated hyperperistaltic segments can be seen proximal to the strictured segments. Aneurysmal dilation and sacculation may be seen proximal to the involved segments.

Fistula appear as linear bands of varying echogenicity ( Fig. 41-11 ), extending from the gut to the bladder, another segment of bowel, or bladder. Gas within the fistula will appear echogenic and may show the so-called ring-down artifact. An empty or partially closed tract will appear as a hypoechoic segment of bowel or bladder. Abscesses on ultrasound manifest as a fluid-filled or complex mass, which may contain gas.

Recently, in an animal model, ultrasound elastography-derived shear wave velocity was found to help distinguish acutely inflamed from fibrotic bowel. Much work needs to be performed to determine whether this technique can distinguish mixed fibrostenotic and active inflammatory disease from pure active inflammatory disease or fibrostenotic disease.
Magnetic Resonance Imaging
Magnetic resonance imaging (see Chapter 40 ) has become a well-established technique in evaluating patients with known or suspected Crohn’s disease by virtue of its ability to help confirm the diagnosis; localize lesions; assess their severity, extent, and inflammatory activity; and identify extraintestinal complications that may require surgical intervention. MR enteroclysis is an emerging diagnostic tool that combines the advantages of conventional enteroclysis and MRI. However, MR enterography (MRE) is the most practical method of evaluating patients with Crohn’s disease.
MRE has two major advantages over CTE. No ionizing radiation is used and, as a result, multiple pulse sequences with and without contrast enhancement can be performed. Static imaging can help identify wall edema and lymphatic distention, and contrast-enhanced imaging can be performed long after the injection has been given, potentially allowing for further characterization. The major disadvantage is a long examination time, requiring relatively long breath holding. Furthermore, magnet time is at a premium in many institutions and is used for many other disease states. Bowel motion remains a problem; glucagon and hyoscyamine (Levsin) are the only antiperistaltic agents approved in the United States (Buscopan or hyoscine butylbromide is an excellent agent but only approved in countries other than the United States). Finally, spatial resolution, even with 3-T magnets, does not approach the resolution of MDCT with its near-isotropic imaging. Nevertheless, MRE is equivalent to CTE for the evaluation of Crohn’s disease.
Adequate distention of the bowel lumen is ideal in MRE because collapsed bowel loops can potentially hide lesions or mimic pathology. However, in general, the small bowel affected by Crohn’s disease can almost always be detected unless there is significant bowel motion or no distention of a large amount of small bowel. Different methods have been used to achieve adequate distention. MR enteroclysis provides distention of the entire small bowel, visualizes mucosal abnormalities, and can show functional information about small bowel mobility.
Regardless of the pulse sequence, it is important to obtain imaging in orthogonal planes, often axial and coronal. These planes should be obtained in all patients on static and postcontrast scans. As with CTE, disease presence, strictures, and especially fistulae and sinus tracts are best elucidated with orthogonal imaging.
Many T2-weighted pulse sequences are available on modern scanners. New, fast, T2-weighted spin-echo sequences can be obtained in a single breath-hold. These include T2-weighted, half-Fourier rapid acquisition with relaxation enhancement (RARE), turbo spin-echo (TSE), fast spin-echo (FSE), half-Fourier acquisition single-shot turbo spin-echo (HASTE), single-shot turbo spin-echo (SSTSE), EXPRESS, and the newest sequence, called BLADE, which uses periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER). These T2-weighted sequences are obtained with and without fat saturation. Fat saturation helps identify and characterize increased T2 signal in abnormal loops of bowel (increased T2 signal in the wall can result from increased fluid from edema or lymphatic distention or from fat deposition). T1-weighted, gadolinium-enhanced, spoiled gradient-echo (SGE) sequences are also important in evaluating the location, extent, and severity of Crohn’s disease. T1-weighted gadolinium-enhanced MRI has been the most investigated pulse sequence in Crohn’s disease. A variety of 2D and 3D techniques are available; the most common use a fast, 3D, fat saturated gradient-echo sequence acquired in a single breath-hold lasting 15 to 20 seconds. These sequences have a variety of names, such as VIBE, FAME, THRIVE, and 3D QUICK. T1 acquisition in regard to contrast enhancement varies, with acquisition commonly at 30, 60, and 90 seconds postinjection start. More delayed imaging of up to 8 minutes postcontrast may be helpful.
Multiple coronal, true fast imaging with steady-state precession (FISP), cine motility sequences have been reported to differentiate between a high-grade and low-grade partial small bowel obstruction. These sequences give an MR “fluoroscopic” depiction of peristalsis above a stricture, showing the progressive dilation of bowel proximal to the stricture. Some believe that static imaging may not be able to make this distinction. However, in our experience, most significant strictures (predominantly fibrostenotic) demonstrated dilated loops of small bowel (>3 cm) proximally.
Recently, in an animal model of Crohn’s disease, magnetization transfer helped detect fibrosis.
Computed Tomography
There are three major CT techniques (see Chapter 38 ) used for the evaluation of Crohn’s disease of the small bowel—conventional positive intraluminal contrast studies, CT enterography (CTE), and CT enteroclysis. Conventional CT using positive intraluminal contrast agents is generally reserved for postoperative patients in whom a bowel leak is suspected. In a patient with an acute exacerbation of symptoms, one might argue that identifying an abscess is the most important question. Thus, conventional CT with positive oral agents should be used. However, many patients with acute symptoms do not have an abscess but have exacerbation of the disease, obstruction or progressive stricture formation with or without fistulization. We have found that CT enterography, even in these acutely ill patients, can confidently distinguish an abscess using neutral oral contrast agents. CT enteroclysis is a rarely used technique and is reserved for those institutions in which it is performed routinely. Finally, the assessment of mural enhancement is hindered by the presence of positive intraluminal contrast because this contrast obscures inner wall hyperenhancement.
CT enterography uses MDCT with a narrow section thickness and reconstruction interval, vis-à-vis (IV) contrast material, and large volumes of neutral contrast agent to distend the lumen in an effort to improve the detection of small bowel inflammation and extracolonic complications. CT can be performed during the enteric phase (45 seconds after injection) and portal venous phase (70 seconds after injection). Regardless of the technique used, there is no significant difference in detecting Crohn’s disease. Jejunal attenuation is greater than ileal attenuation, and collapsed bowel loops demonstrate greater attenuation than distended bowel loops. CT enteroclysis uses contrast material infused through a nasojejunal tube. Both positive (dilute barium) and neutral contrast (water, methylcellulose, VoLumen) agents may be administered. CT enteroclysis uses the same technique as standard fluoroscopic enteroclysis, except that imaging is performed with contrast-enhanced MDCT, as in CT enterography. All MDCT datasets should be reconstructed in at least two planes, typically axial and coronal. Occasionally, sagittal or oblique planes are helpful in identifying fistulae.
Every effort should be made to limit radiation exposure in patients, especially if they have had multiple CT scans in the past. Several studies have shown that some Crohn’s patients can receive large cumulative doses (>100 mSv) over the course of their disease, and often are examined with CT two to three times a year. In one series, encompassing a 15-year period, the mean ionizing radiation dose was 36.1 mSv. Over the entire study period, there was an increasing use of CT, and although CT accounted for only 16.2% of all imaging studies, it accounted for 77.2% of the radiation dose. Also, in this study, the total ionizing radiation exceeded 75 mSv in 15.5% of patients. Patients who receive higher doses have onset of disease before 17 years of age, have upper GI tract or penetrating disease, require IV steroids or infliximab, or have had multiple surgeries. There is recent evidence that radiation exposure from CT scans in children results in an increased risk of brain tumors and leukemia. Most pediatric centers almost exclusively use MRE or ultrasound as a means of evaluating their patients.
Efforts to reduce the dose from MDCT are ongoing and include alterations in kVp and mAs in relation to body habitus, weight, body mass index (BMI), and altering the scan pitch, as well as applying new reconstruction techniques, generally termed iterative reconstruction, to initial lower dose images. Dose reductions from a CT scan are typically 15 to 20 mGy but can successfully be reduced to less than 10 mGy, and even below 5 mGy, have been achieved. However, the most substantial reductions in dose have been in patients who weigh less than 160 pounds. It remains to be seen whether sub-mSv imaging is possible without data loss. Crohn’s disease identification is a high-contrast issue with CT (i.e., identifying a process with a higher attenuation compared with background). To date, low-contrast objects (an object of lower attenuation compared with background) are lost with lower dose MDCT, even with any of the known iterative reconstruction techniques, including model-based iterative reconstruction. To achieve a substantial reduction in dose from CT, radiologists may have to accept a lower sensitivity in detecting low-attenuation lesions in the liver, which are the low-contrast entities that occur most frequently in a patient population.
The most effective strategy for reducing ionizing radiation exposure in patients with Crohn’s disease is to shift imaging to MRE from CTE. Our strategy is first to perform CTE on all patients with Crohn’s disease if they have not had one performed. This serves as the baseline examination, establishing the location and extent of disease. The study is fast, the spatial resolution is superb, and there is little to no bowel motion artifact. We favor CTE or MRE as the initial study because bowel motion remains a problem for MRE in the United States. Furthermore, the spatial resolution of MRE is still not equivalent to that of CTE. As a result, the risk of missing disease on an initial MRE image is relatively high. However, every other examination, except for patients who are acutely ill or perioperative, should be MRE. Acutely ill patients should be examined with CTE, especially if abscess detection and potential drainage is relatively likely. Once detected, the abscess can be rapidly drained using CT guidance. Postoperative patients should be examined after the ingestion of positive oral contrast medium and the administration of rectal contrast medium if there is a colonic anastomosis, because a leak may be present.
Nomenclature for CT and MR Enterography Findings
In an effort to measure the impact of CTE and MRE in the care of Crohn’s patients, interested radiologists in the Abdominal Radiology Society (ARS), Crohn’s Disease-Focused Panel are in the process of developing a nomenclature to describe the findings of Crohn’s disease and how to interpret those findings. Tables 41-1 and 41-2 summarize this work, which should be considered a work in progress. The ultimate goal is to have international input and approval from radiologic, gastroenterologic and surgical societies. The purpose of a common nomenclature is to define and standardize the terms used to describe the findings and the conclusions that can be drawn from those findings. Ultimately, these terms and conclusions will be essential in measuring outcomes in regard to treatment and the impact of imaging on predicting treatment. These terms will be used here in the subsequent discussion of the findings of MRE and CTE.
| Findings | Description |
|---|---|
| WALL FINDINGS | |
| WALL HYPERENHANCEMENT | Increased attenuation and signal intensity on contrast-enhanced scan in noncontracted segment; compare with nearby small bowel segments. |
| Stratified (bilaminar or trilaminar) | Inner wall hyperenhancement or halo; no clinically significant difference between the two patterns |
| Homogeneous | Transmural hyperenhancement |
| Normal | |
| Asymmetric | Asymmetric or patchy hyperenhancement. often along mesenteric border with relative sparing along the antimesenteric border |
| WALL THICKENING | |
| 3-4 mm |
| 5-7 mm |
| >7 mm |
| Intramural edema | Hyperintense T2 signal; only on MRE (preferably fat suppressed); cannot comment on CTE |
| LUMINAL NARROWING | Narrowed diameter in a noncollapsed loop, with or without mural hyperenhancement |
| STRICTURE | Luminal narrowing + upstream dilation (see below) |
| UPSTREAM DILATION | Bowel lumen >3 cm, unequivocal segmental dilation of small bowel proximal to narrowed segment or small bowel feces sign above narrowed segment |
| <4 cm |
| 4-5.9 cm |
| >6 cm |
| Ulcer | Wall defect that does not extend outside bowel wall in a thickened wall |
| Sinus tract | Wall defect that extends outside lumen but not to adjacent organs (usually accompanied by angulation and fixation of adjacent bowel and/or tethering of bowel or urinary bladder) |
| Simple fistula | Single tract; affected loops often angulated and tethered; describe origin and termination location |
| Complex fistula | Multiple tracts; asterisk appearance; +/interloop abscess or inflammatory mass (phlegmon) |
| MESENTERIC FINDINGS | |
| Vasa recta distention | Also known as the comb sign in the literature; inflammed bowel loop with dilation of mesenteric vessels larger than adjacent to bowel loops |
| Fibrofatty proliferation | Increased fat adjacent to abnormal bowel, displacing bowel loops; usually along mesenteric border, unless perirectal; often associated with mesenteric border inflammation; if perirectal, then circumferential |
| Perienteric edema, inflammation | Increased attenuation (CT) or high T2 signal (MR) in mesenteric fat adjacent to abnormal bowel loops |
| Inflammatory mass | Ill-defined, masslike process of soft tissue attenuation and signal intensity (not water attenuation and signal intensity; also known as a phlegmon) usually associated with penetrating disease, such as complex fistula and inflammatory stranding in the mesenteric fat |
| Abscess | Mesenteric or peritoneal fluid collection with rim enhancement or internal gas |
| Amenable to percutaneous drainage | Well-formed collection >3 cm, accessible to intervention |
| Not amenable to percutaneous drainage | Not well formed, or <3 cm, or not accessible to intervention because of bowel, bony, or vascular structures |
| Adenopathy | >1.5 cm in short axis |
| Likely inflammatory | If <2 cm in short axis |
| EXTRAINTESTINAL FINDINGS | |
| Sacroiliitis | |
| PSC | Discontinuous, intrahepatic bile duct visualization and/or extrahepatic ductal wall thickening without significant upstream dilation |
| Venous thrombus, thrombosis (occlusive) | Mesenteric, portal, IVC, iliac, femoral, gonadal |
| Nephrolithiasis and cholelithiasis | |
| Avascular necrosis | Femoral head most common; if present, describe articular collapse |
| Findings | Description |
|---|---|
| Active inflammatory small bowel Crohn’s disease (luminal narrowing present or absent) | Wall hyperenhancement in noncontracted small bowel segment; wall thickening, mesenteric changes are variable but often present |
| Quiescent or inactive small bowel Crohn’s disease | Absent wall hyperenhancement, ± wall thickening, no mesenteric changes except fatty proliferation |
| Mixed fibrostenotic and active inflammatory small bowel Crohn’s disease (a controversial classification to some; see text) | Stricture, wall thickening, wall hyperenhancement, luminal narrowing with upstream dilation; most strictures have inflammatory and fibrotic components |
| Penetrating Crohn’s disease (added in addition to determination of active or mixed Crohn’s disease) | Sinus tract and/or fistula; inflammatory mass; abscess; free perforation; associated with mixed fibrostenotic and active inflammatory small bowel Crohn’s disease or active inflammatory small bowel Crohn’s disease |
| Fibrostenotic small bowel Crohn’s disease | Wall thickening, no wall hyperenhancement, absent mural edema (MRE only), no mesenteric changes, lumen narrowed, with upstream dilation |
Computed Tomography and Magnetic Resonance Enterography Findings of Small Bowel Crohn’s Disease
Both CTE and MRE identify the transmural, extramural, and mesenteric manifestations of small bowel Crohn’s disease. Historically, investigators have described findings as mucosal, submucosal, serosal, or extramural-mesenteric. However, in many cases, the mucosa may be entirely destroyed, replaced by acute and chronic inflammatory cells. Furthermore, the precise anatomic location of the finding may not actually be known. Therefore, we think it best to avoid the use of these specific terms and prefer to use terms that are more generic and descriptive, rather than anatomic (see Table 41-1 ). Finally, because the findings on CTE and MRE are similar or identical, we will combine the discussion into one section.
Wall Findings
Hyperenhancement.
One of the most consistent MRE/CTE findings is wall hyperenhancement. This is defined as increased signal intensity or attenuation on contrast-enhanced scans in a noncontracted segment of bowel. Comparison with adjacent or nearby small bowel segments is helpful in making this differentiation. Furthermore, accompanying mesenteric findings, such as vasa recta distention or fibrofatty proliferation, are almost always present. Hyperenhancement may be layered or stratified into two patterns, bilaminar and trilaminar. In the bilaminar pattern, only the inner wall hyperenhances ( Figs. 41-12 and 41-13 ). This is often referred to in the literature as mucosal hyperenhancement. Because the mucosa may be destroyed, we prefer to describe rather than conclude that the mucosa is present. In the trilaminar pattern, there is inner and outer wall (sometimes referred to as serosal) hyperenhancement, with the intervening wall relatively hypoenhancing, giving a halo effect ( Fig. 41-14 ). This pattern is much more commonly seen in MRE; it may very well be present in CTE but is not as well identified because of poorer contrast resolution compared with MRE. There is likely no clinically significant difference between bilaminar and trilaminar hyperenhancement. Both are present with active inflammation. Hyperenhancement may be homogeneously transmural ( Figs. 41-15 and 41-16 ), asymmetric, or patchy ( Figs. 41-17 and 41-18 ). The hyperenhancement may at times be very subtle; viewing with narrow windows facilitates identification (see Fig. 41-18 ). When asymmetric, the pseudosacculation of the nonaffected segment is sometimes present (see Fig. 41-17 ).












