Fig. 30.1
Superior axial illustration of the pelvic diaphragm demonstrates the relationship of the levator ani, composed of the puborectalis, pubococcygeus, and iliococcygeus, and the coccygeus muscles to the female urethra, vagina, and rectum. The muscles of the urogenital diaphragm form the pelvic floor
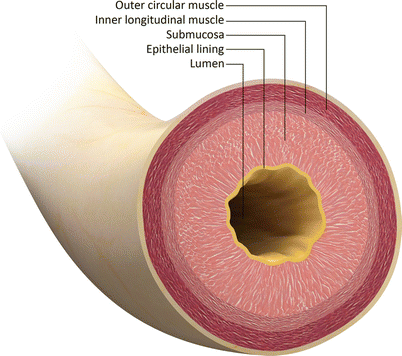
Fig. 30.2
Transverse schematic through the urethra shows the concentric anatomic layers. The urethral lumen is enclosed by the epithelial mucosa. Submucosa surrounds the mucosal layer. The external layer is composed of smooth muscle: an inner longitudinal layer and an outer circular layer
Imaging Techniques of the Female Urethra
VCUG and retrograde double-balloon positive-pressure urethrography are the classically utilized techniques in evaluating female urethral pathology. In more recent years, US and MR imaging have supplanted these conventional techniques.
Ultrasound
Transvaginal, transperineal, and transurethral US allow assessment of the urethra, bladder, and pelvic organs in a cost-effective manner without radiation. For transvaginal US, a 5–9-MHz curved array transducer is placed approximately 1–2 cm into the vagina. For transperineal US, a 5–10-MHz linear-array transducer is placed on the perineum between the labia. For transurethral US, a 12.5-MHz endoluminal transducer is placed directly into the urethra [7]. Patients are typically studied in a lithotomy position with a partially filled bladder. Transperineal sonography enables dynamic evaluation of the perineum and urethra at rest and with valsalva to evaluate for abnormal movement of the urethra with increased abdominal pressure.
Endoluminal sonography has numerous advantages, including the absence of radiation and contrast exposure; avoidance of urethral catheterization for luminal contrast injection; relative ease of imaging in axial, sagittal, coronal, and off-axis orientations; ability to perform real-time multiplanar imaging during straining; relative ease in distinguishing between solid and cystic lesions; and the evaluation of periurethral processes [8]. Disadvantages include imaging results based on operator dependence and experience, difficulty in differentiating periurethral cysts from small urethral diverticula, difficulty in differentiating complex cystic diverticula from solid masses within diverticula, and decreased diagnostic confidence in detecting ostia of small diverticula [9]. Furthermore, transurethral sonography currently has limited utilization and availability due to the high cost of dedicated equipment and a limited field of view [7].
At US, the female urethra appears as a tubular hypoechoic structure extending from the bladder neck to the vaginal vestibule, coursing inferior to the pubic symphysis and anterior to the vagina (Fig. 30.3). Anechoic urine may be seen centrally in the urethra in patients with incontinence or pelvic organ prolapse.
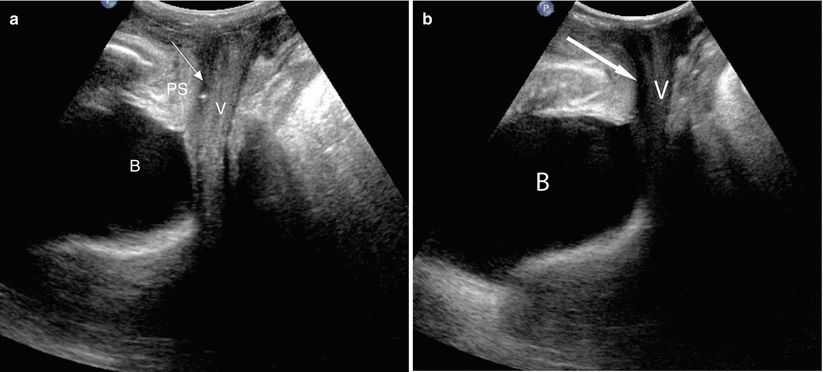

Fig. 30.3
Female urethral anatomy at US. Longitudinal translabial US image in a patient with a normal urethra (a) shows a thin hypoechoic tubular structure (arrow) extending from the bladder (B) to the vestibule. It travels inferior to the pubic symphysis (PS) and anterior to the vagina (V). Longitudinal translabial US image in an incontinent patient (b) demonstrates a tubular urethra filled with hypoechoic urine (arrow). Whereas the urethral walls of continent females are collapsed, hypoechoic or anechoic urine can distend the urethral walls of incontinent patients
Magnetic Resonance Imaging
MRI has higher sensitivity and specificity than VCUG or retrograde urethrography in the detection of urethral and periurethral disorders [9–12]. MRI may help detect noncommunicating urethral diverticula or periurethral disease and structural anomalies that would not be visible with the traditional modalities. Because of its many inherent advantages including lack of radiation, noninvasiveness, high inherent tissue contrast, increased signal to noise ratio, high spatial resolution, ability for 3D imaging, and multiplanar capability, MRI has become the imaging modality of choice for the diagnosis and preoperative planning in female urethral and periurethral disease.
Endocavitary (endovaginal or endorectal) coils may be used for specialized indications. These provide the highest-resolution images of the female urethra and periurethral anatomy [10]. The use of an endovaginal coil with a pelvic phased-array coil has been shown to allow better soft-tissue differentiation of the periurethral region than other combinations of endocavitary coils and phased-array coils [13].
A standard urethral MR protocol on a 1.5 or 3T system should be performed with a phase array coil for reception and may include the following sequences: axial multishot fast spin-echo (SE) T2-w with fat saturation, axial single-shot fast SE T2-w with and without fat saturation, sagittal and coronal single-shot fast SE T2-w without fat saturation, axial dual echo gradient-echo T1-w sequence, and axial opposed phase T1-w with fat saturation. In addition, small field-of-view images may be obtained through the urethra with axial, sagittal, and coronal sequences (fast imaging with steady-state precession).
The normal female urethra on T2-w and gadolinium-enhanced T1-w MRI has been described as target like on axial series due to the alternating signal characteristics of the different zones. Four concentric rings corresponding to the concentric anatomic layers discussed above can be seen on axial and sagittal T2-w images: an outermost layer of longitudinal smooth and circular striated muscle and serosa (hypointense), a middle submucosal layer of vascularized connective tissue and smooth muscle (hyperintense), an inner mucosal layer of epithelial cells (hypointense), and an innermost lumen of hyperintense urine or secretions that may or may not be seen (Fig. 30.4) [6, 14]. On post gadolinium T1-w images, mucosa enhances more than other layers in a normal urethra.
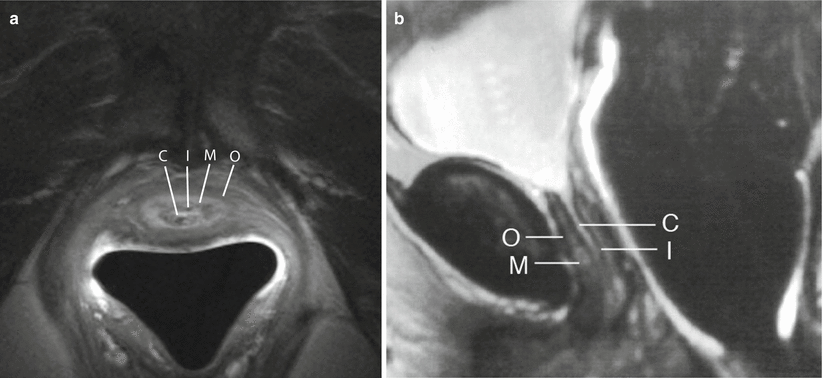

Fig. 30.4
Zonal anatomy of the normal female urethra. Axial (a) and sagittal (b) T2-w MR images demonstrates a high-signal-intensity central zone (C) representing luminal urine or secretions, a low-signal-intensity inner mucosal layer (I) representing epithelial cells, a high-signal-intensity middle submucosal layer (M) representing vascularized connective tissue, and a low-signal-intensity outer muscular layer (O) representing smooth muscle. (a) is used with permission from [70]
Diagnostic Algorithms
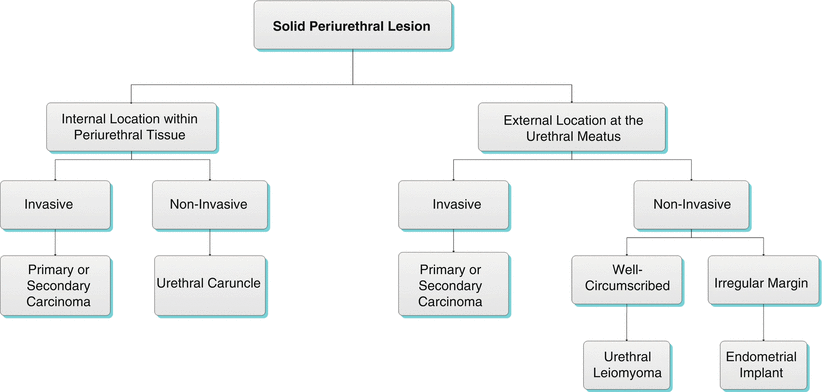
Algorithm 30.1 Solid periurethral lesion
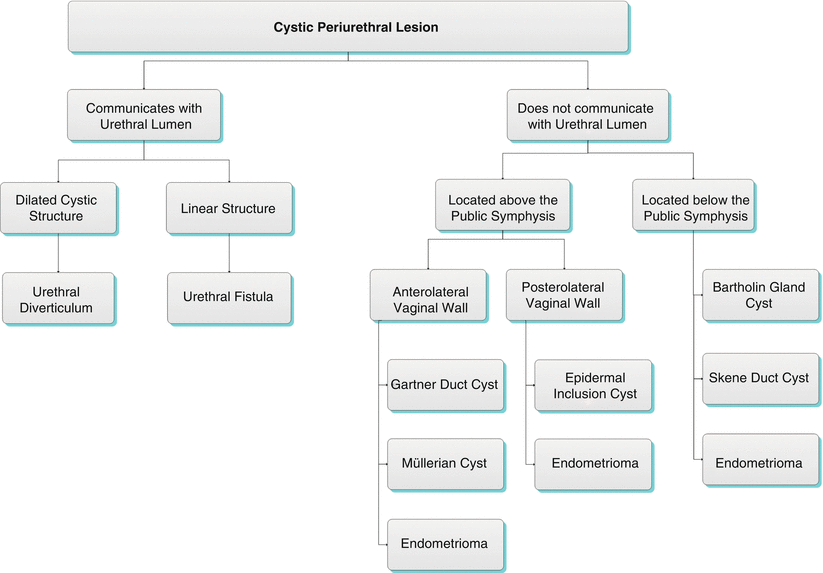
Algorithm 30.2 Cystic periurethral lesion
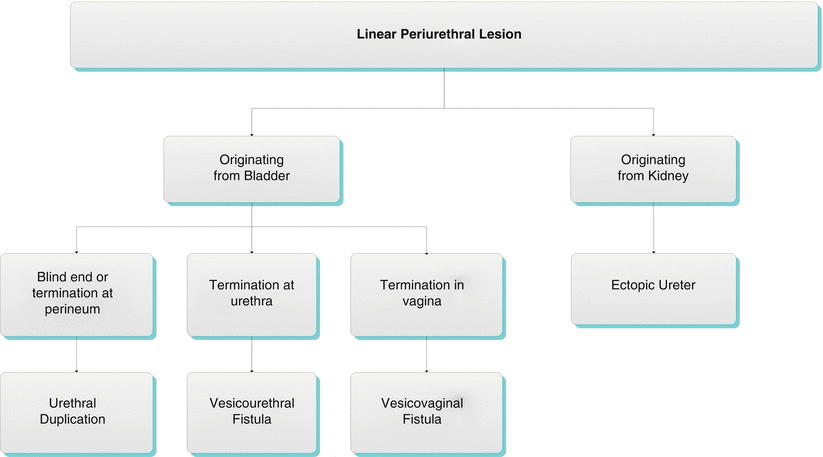
Algorithm 30.3 Linear periurethral lesion
Pathologic Classification
Pathologic classification table of urethral lesions
Congenital | Acquired/Iatrogenic | Inflammatory/Infections | Benign proliferation or neoplasm | Malignant neoplasm |
|---|---|---|---|---|
Urethral duplication | Urethral diverticulum | Urethral fistula | Urethral caruncle | Primary urethral carcinoma (squamous cell, transitional cell, adenocarcinoma) |
Ectopic ureter to urethra | Urethral fistula | Bartholin gland cyst | Urethral leiomyoma | Secondary urethral carcinoma (direct extension from adjacent primary, hematogenous metastases) |
Gartner duct cyst | Epidermal inclusion cyst | Skene duct cyst | Perineal – vulvovaginal endometrioma | |
Müllerian duct cyst | Pelvic organ prolapse (urethral hypermobility, urethrocele, cystocele, cystourethrocele) |
Benign Urethral Pathology
Urethral Diverticulum
Urethral diverticula are acquired protrusions of the urethral mucosa into the periurethral fascia. The epithelial mucosal lining of a diverticulum is similar to urethral mucosa, and communication with the urethral lumen is usually maintained via a small neck [1, 15]. Although prevalence has been reported to be between 0.6 and 6 % of all women, the true prevalence of urethral diverticula is likely underestimated due to misdiagnosis and lack of symptoms [15]. Urethral diverticula have no racial predilection and can occur at any age, although they are most common in the third to fifth decades.
The vast majority of urethral diverticula are acquired, with the most widely accepted theory involving occlusion of a paraurethral duct with obstruction of drainage resulting in abscess formation and, ultimately, rupture into the urethral lumen (Fig. 30.5). The outpouching epithelializes over time and becomes a true diverticulum lined with urothelium [16]. Chronic urethral infections, typically with Escherichia coli, are the most common cause of diverticula formation. Other infectious etiologies include gonococcus and Chlamydia species. Birth trauma, urethral instrumentation, and surgical trauma are less common acquired etiologies. Congenital etiologies have been postulated and include an origin from cloacogenic rests, Gartner duct remnants, or incorrect union of primordial urogenital sinus folds [15].
Urethral diverticula vary in size, shape, neck width, and location along the urethral length. They may be spherical, horseshoe-shaped around the urethra, dumbbell-shaped, or circumferentially surrounding the entire urethra. Diverticula may contain septations or loculations. Infected or inflamed diverticula may erode into adjacent structures, such as the vagina, leading to fistula formation. Microscopically, the diverticulum is lined by a continuation of the urethral mucosa, with either transitional cell epithelium or stratified squamous epithelium. There may be reactive and inflammatory changes to the mucosa and squamous or adenomatous metaplasia [17]. A small subset of diverticula lack a neck and are classified as intraurethral mural diverticula or noncommunicating diverticula. These lesions are thought to represent the initial stage of true urethral diverticulum formation and may be symptomatic [18].
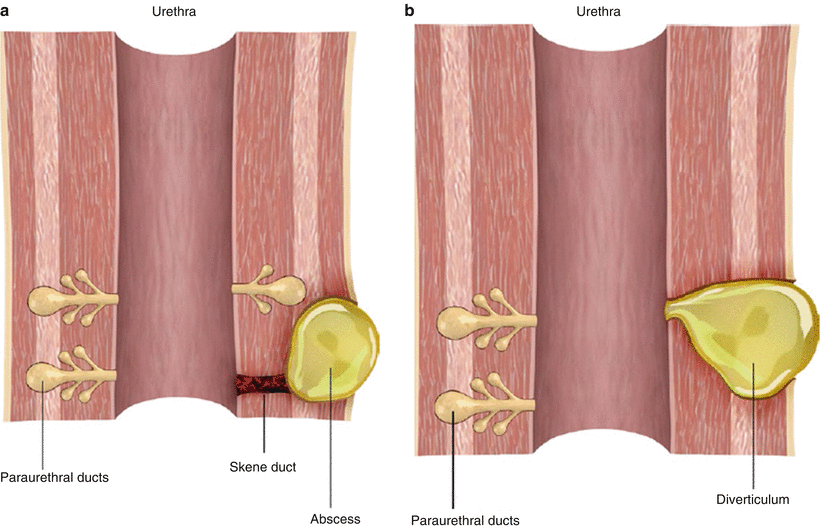

Fig. 30.5
Illustration of the formation of a female urethral diverticulum. (a) Obstruction of a normal Skene duct leads to paraurethral gland obstruction and dilatation with accompanying inflammation, infection, and abscess formation. (b) The paraurethral gland abscess ruptures into the lumen of the urethra, forming a communicating diverticulum. The outpouching epithelializes with urothelium over time
The most specific presentation of a symptomatic urethral diverticulum includes dysuria, dyspareunia, and postvoid dribbling. However, most patients present with less specific symptoms. The differential diagnosis for this nonspecific clinical presentation is vast and includes urethritis, cystitis, infected or inflamed periurethral cysts, periurethral fibrosis, urethrocele, urethral carcinoma, and endometrioma of the urethra. Most commonly, no etiology is diagnosed and the patient carries a diagnosis of “chronic pelvic pain.” Physical examination is of limited utility because urethral diverticula may not be palpable or tender, may present as a discrete lesion or diffuse fullness along the length of the urethra, or may mimic vaginal wall cysts or lesions [15]. Studies have shown that diagnosis is delayed due to these challenges and there is a lag of up to 9.5 months from time of presentation to diagnosis of urethral diverticulum [1].
On VCUG and retrograde urethrography, a urethral diverticulum may appear as a contrast-filled spherical or ovoid outpouching of variable size and shape posterolateral to or surrounding the urethra. However, these modalities are limited in diagnosing urethral diverticula because the lesion may not fill with contrast due to a narrow neck, septations, or obstruction by inflammatory debris or calculi [17].
US demonstrates an anechoic cystic lesion adjacent to or surrounding the urethra. US can differentiate between cysts with septations and/or intraluminal debris or mass as opposed to VCUG or retrograde urethrography which can only give the appearance of a filling defect in all cases. An abnormal focus of echogenicity within the cyst with or without posterior acoustic shadowing suggests the presence of a calculus, hemorrhagic or proteinaceous debris, or neoplastic growth within the diverticulum. Color Doppler can give additional information about vascularity of diverticular wall and solid contents in cases of neoplasm [19]. Transvaginal or perineal US may not be able to demonstrate diverticula with small or irregular necks, which would require intraurethral US or MRI for definitive diagnosis.
At MRI, urethral diverticula are most commonly seen to arise from the posterolateral wall of the mid urethra behind the pubic symphysis. A simple urethral diverticulum is round or oval and usually located lateral or posterior to the urethra. A U-shaped diverticulum extends partially around the urethra while circumferential diverticula extend completely around the urethra, often having a “saddlebag” appearance (Fig. 30.6) [8].
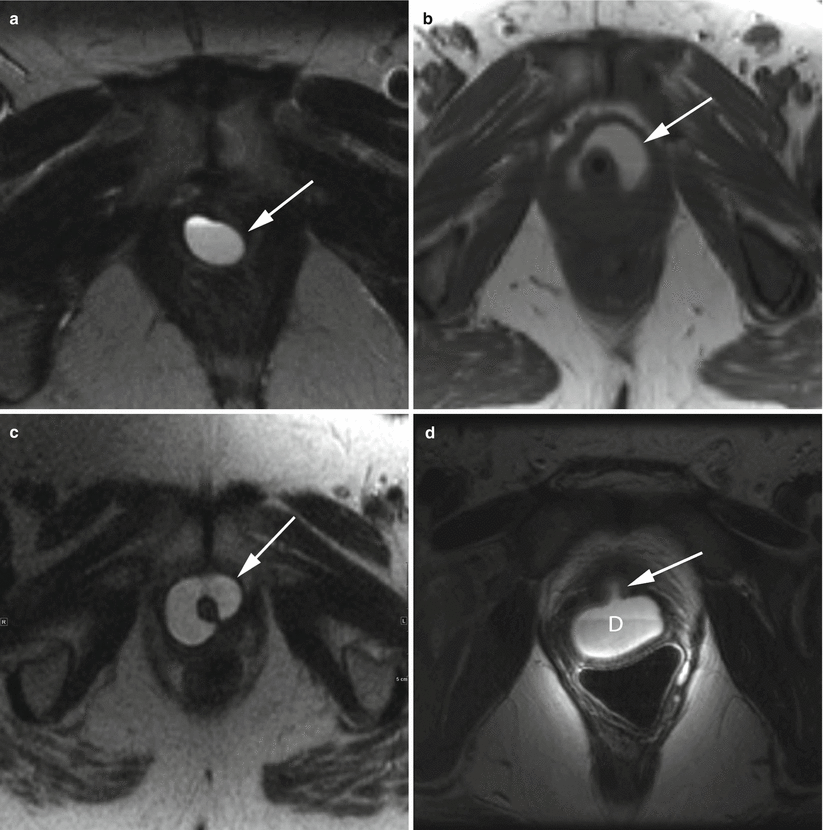

Fig. 30.6
Variable appearances of urethral diverticula. Axial T2-w MR images demonstrate a simple diverticulum (a), a U-shaped diverticulum (b), and a near-circumferential diverticulum (c) (arrows). In all cases, the diverticulum is a well-circumscribed hyperintense collection adjacent to the urethra. Axial T2-w image using an endovaginal coil (d) shows a posteriorly located diverticulum (D) with a visible neck (arrow) in communication with the urethral lumen. This diverticulum contains a fluid-debris level. (d) is used with permission from [70]
Urethral diverticula demonstrate fluid signal, appearing hyperintense on T2-w sequences and hypointense on T1-w sequences (Fig. 30.7), without significant contrast enhancement.
Diverticula containing hemorrhagic or proteinaceous contents appear hyperintense on T1-w and hypointense on T2-w images (Fig. 30.8). Inflamed urethral diverticula may also demonstrate heterogeneous signal on T1-w images and marked hyperintensity on T2-w images with a possible fluid-fluid level [6].
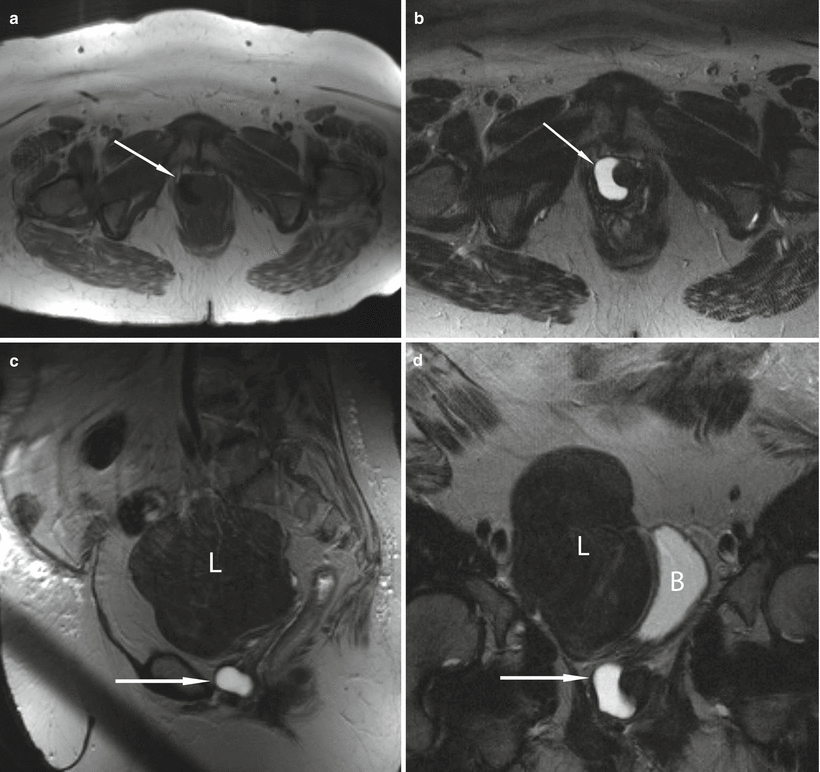
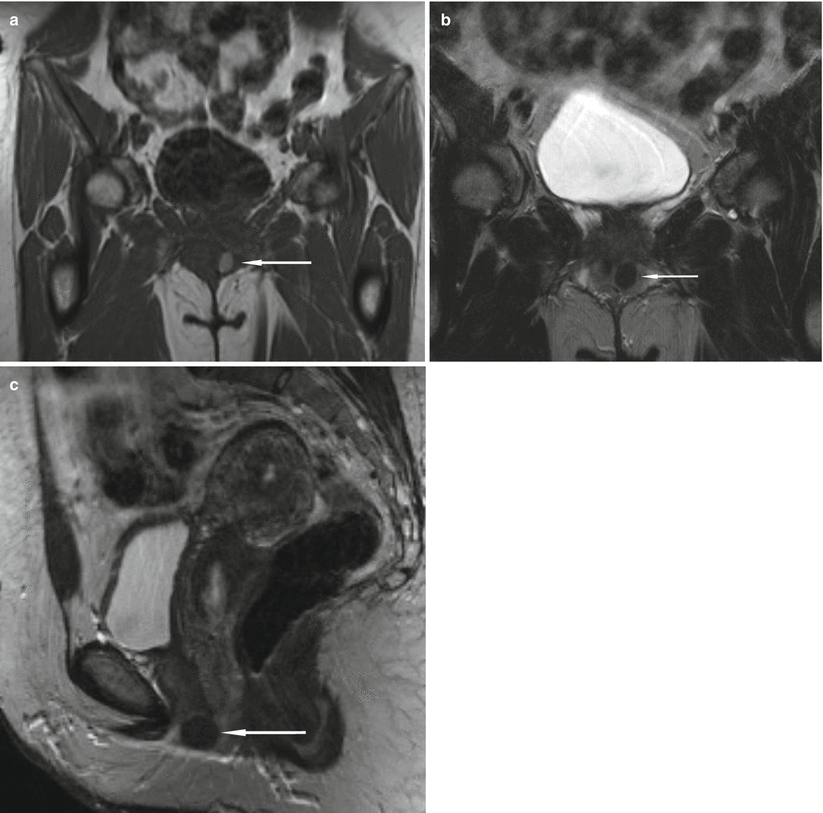

Fig. 30.7
Simple urethral diverticulum in a 50-year-old woman with a 6-month history of urinary urgency. Axial T1-w (a) and axial (b), sagittal (c), and coronal (d) T2-w images demonstrate a T1 hypointense and T2 hyperintense cystic structure (arrows) at the right aspect of the midurethra at the level of the pubic symphysis. A large uterine leiomyoma (L) which displaces the urinary bladder (B) to the left is incidentally noted

Fig. 30.8
Urethral diverticulum with hemorrhagic or proteinaceous contents in a 39-year-old woman with refractory urinary tract infections. Coronal T1-w (a), coronal T2-w (b) and sagittal T2-w (c) MR images demonstrate a single circumferential T1-hyperintense, T2-hypointense lesion surrounding the distal urethra (arrows), consistent with a urethral diverticulum containing hemorrhagic or proteinaceous products
For preoperative planning, the diverticulum should be described with attention to its location, size, number, configuration, possible sac contents, mass effect, and position of the neck. The neck is important to describe as it must be resected to prevent recurrence [5]. Radiologists may use a “clock face” template with axial MRI to more clearly convey to the surgeon the exact location of the urethral diverticulum and neck (Fig. 30.9).
Urethral diverticula form a focus of urinary stasis and therefore are a nidus for recurrent infection, stone formation, and occasionally malignancy. An infected urethral diverticulum is suggested at MRI by the presence of heterogeneously increased signal intensity on T1-w images and increased signal intensity of the diverticular wall on T2-w images. The wall or neck may be thickened and demonstrate enhancement after intravenous contrast. There may be adjacent inflammatory changes in the periurethral fat.
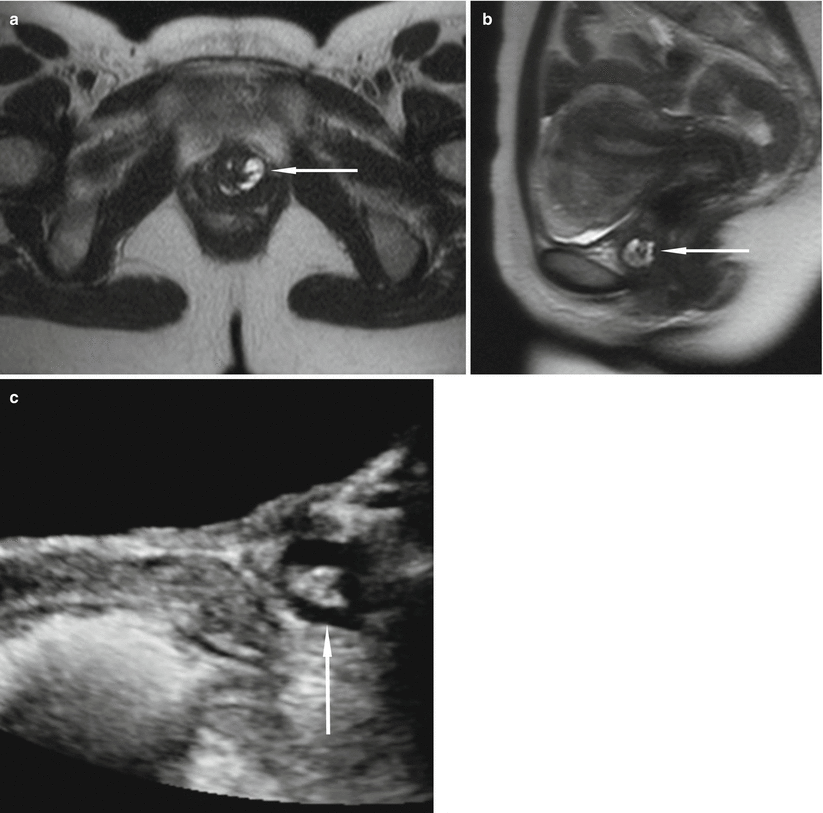

Fig. 30.9
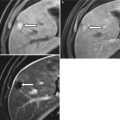
Incidentally discovered multiloculated urethral diverticulum in a 43-year-old woman who complained of chronic urinary tract infections and urinary urgency and frequency. Axial (a) and sagittal (b) T2-weighted HASTE MR images demonstrate a septated U-shaped lesion with fluid signal intensity extending from the 1 o’clock position to the 7 o’clock position along the left posterolateral aspect of the midurethra (arrows). Longitudinal transabdominal US image (c) demonstrates a septated cystic lesion (arrow) at the level of the public bone
Calculus formation occurs in up to 10 % of urethral diverticula and is easily demonstrated on US as an echogenic structure with posterior acoustic shadowing within the urethral diverticulum. At CT, a radiopaque calculus may be visible in a periurethral location, occasionally surrounded by fluid density representing urine within the diverticulum (Fig. 30.10). At MRI, calculi appear as hypointense foci on both T1- and T2-w images.
Rarely, neoplasms are found to arise from urethral diverticula. Although squamous cell carcinoma is the most common malignancy of the female urethra, the majority (60 %) of malignant neoplasms arising from urethral diverticula are adenocarcinomas followed by transitional cell carcinoma (30 %) and then squamous cell carcinoma (10 %) [5]. Appearances vary, but any enhancement in the wall or lumen of the diverticulum requires immediate further workup to distinguish between a benign adenoma and neoplasia. In extreme cases, neoplasms arising from diverticula may present as irregular enhancing heterogeneous solid masses sometimes extending to the soft tissues (Fig. 30.11).
There are various treatment options for symptomatic urethral diverticula including transvaginal and transurethral partial or complete diverticulectomies. Circumferential diverticula greater than 4 cm with delayed diagnosis are most at risk for postoperative complications such as fistula formation and recurrence. For large complex diverticula, complications include stress incontinence and urethrovaginal fistulas [20].
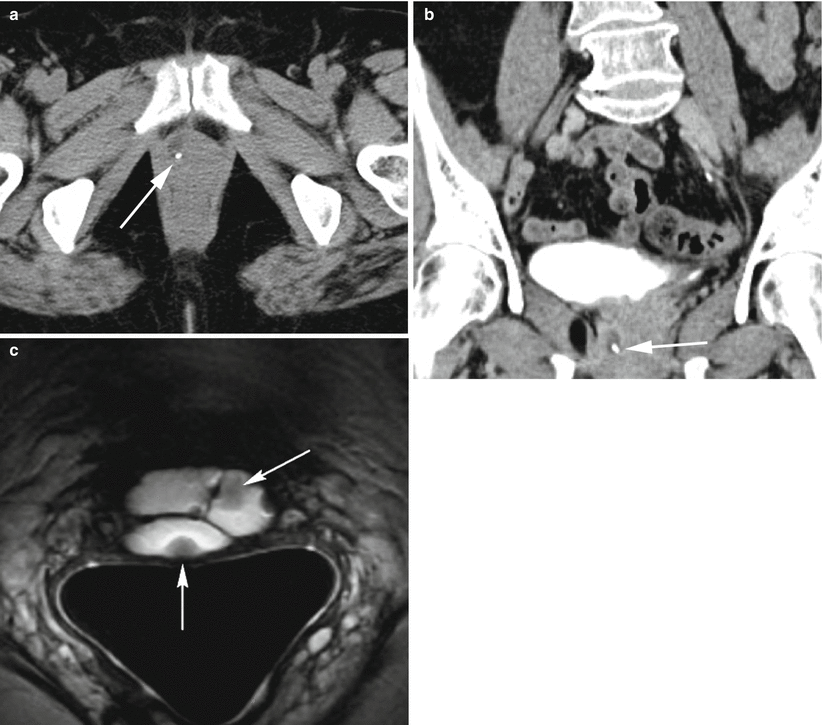
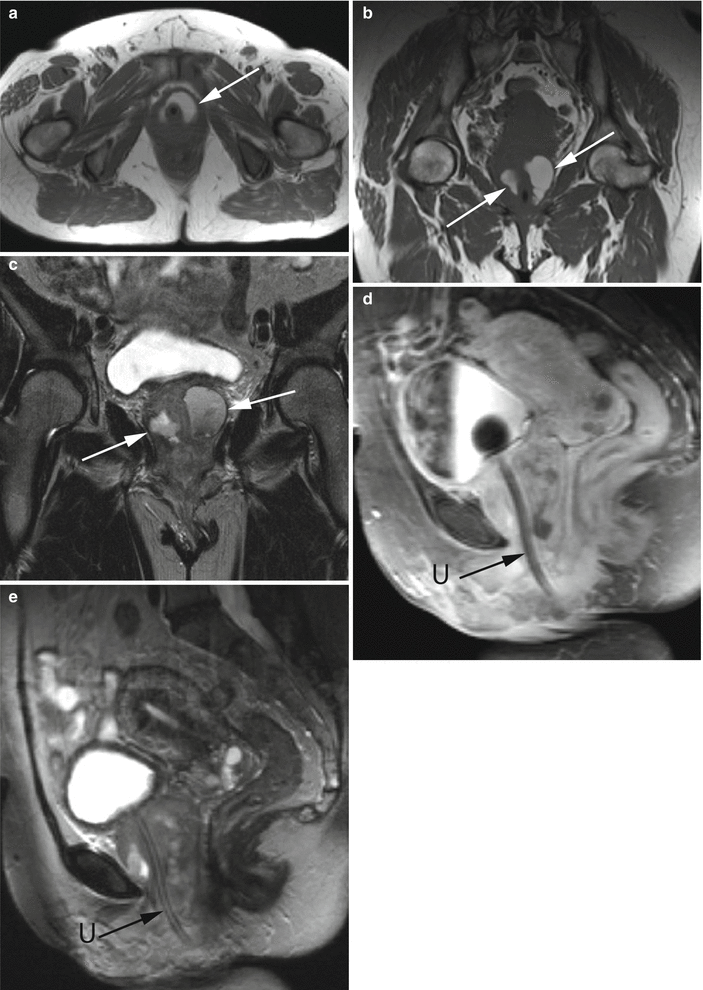

Fig. 30.10
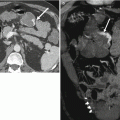
Urethral diverticulum complicated by a calculus. Axial (a) and coronal (b) post-contrast CT images demonstrate a fluid-attenuation cystic structure in the periurethral region with an internal hyperdense calculus representing a stone (arrows) within a urethral diverticulum. Axial T2-w MR image performed with an endovaginal coil (c) in a different patient shows a hyperdense multilocular urethral diverticulum with numerous well-circumscribed internal hypointensities representing stones (arrows)

Fig. 30.11
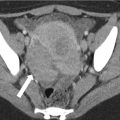
Urethral diverticulum complicated by adenocarcinoma in a 50-year-old woman with hematuria and dysuria. Axial (a) and coronal (b) T1-w images demonstrate a hyperintense saddlebag lesion (arrows) surrounding the urethra consistent with a urethral diverticulum containing proteinaceous or hemorrhagic content. Coronal T2-w (c), sagittal T1-w post gadolinium (d), and sagittal T2-w (e) MR images further demonstrate a large enhancing mass (white arrows in c) surrounding the entire urethra (U). The mass abuts the base of the bladder superiorly and is contiguous with the vagina. Pathologic analysis revealed urethral adenocarcinoma arising within a urethral diverticulum. A Foley catheter is present in the urethra with a balloon in the urinary bladder
Urethral Caruncle
Urethral caruncles are small benign lesions of the posterior margin of the external urethral meatus. In hypoestrogenic postmenopausal women, atrophy of the urogenital tissues occurs and urethral prolapse at the external urethral meatus can result. Chronic irritation to exposed urethral mucosa results in inflammation and fibrosis, increased vascularity, and areas of hemorrhage and necrosis. Thus, urethral caruncles are reactive proliferative lesions and not true neoplasms [21]. In addition to hypoestrogenic urogenital atrophy, chronically increased intra-abdominal pressure or prior pelvic surgery can also contribute to urethral prolapse. In pediatric cases, the pathogenesis may be related to defects in surrounding fascial or muscular layers.
Urethral caruncles are rare and usually occur in either the postmenopausal female population or the pediatric female population. The reported incidence of caruncles has decreased in recent decades due to the increased use of topical vaginal estrogen creams in postmenopausal women. Clinically, the lesion is usually asymptomatic, but some women may have pain, dysuria, and hematuria. There is a visible, palpable mass at the posterior lip of the external urethral meatus in the vestibule. This mass is exophytic and usually polyploid, measuring 1–3 cm. It may be friable or hemorrhagic in appearance. There is a central lumen that connects to the normal urethral lumen. Histology of a caruncle reveals benign hyperplastic squamous epithelium with submucosal vascularity, fibrosis, and inflammation [22]. Occasionally, strangulation may occur, resulting in ischemia and necrosis [23].
US may demonstrate a hypoechoic soft tissue mass at the external urethral meatus. Color Doppler can show blood flow within and around the mass and may be used after conservative treatment to monitor for regression of the lesion, represented by a decrease in vascular flow [24]. At MRI, urethral caruncles manifest as T2-w hyperintense tissue surrounding the external urethral meatus (Fig. 30.12). The tissue enhances on post-contrast series. MRI is also useful to evaluate for regional lymphadenopathy, the presence of which would suggest malignant neoplasm of a soft tissue mass at the external urethral meatus [25].
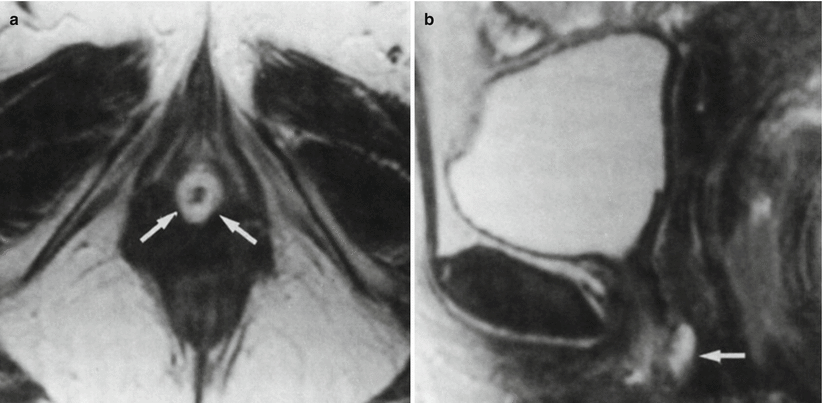

Fig. 30.12
Urethral caruncle in a 65-year-old woman with an external urethral meatus mass. Axial (a) and sagittal (b) T2-w MR images obtained with a pelvic coil demonstrate a high-signal-intensity cuff (arrows) surrounding the external urethral meatus. At pathologic analysis, the lesion proved to be a urethral caruncle. (a) is used with permission from [16]
Conservative treatment in symptomatic patients includes sitz baths and topical estrogen vaginal creams. In cases that do not improve after 4–6 weeks, surgical excision is recommended to exclude neoplasm and prevent complications such as infection, necrosis, and gangrene. Although typically benign, 1–2 % of clinically diagnosed caruncles have been reported to be carcinomas at histology after surgical resection [22, 26].
Urethral Leiomyomas
Urethral leiomyomas are benign mesenchymal tumors composed of smooth muscle that grow in or around the urethra. They are extremely rare and most often occur in premenopausal women, often growing during pregnancy and regressing postpartum, indicating their hormonally affected pathogenesis [27]. Grossly, they appear as focal, encapsulated masses of soft tissue in or around the urethra. They may be sessile or pedunculated and may protrude into the urethral lumen or through the urethral meatus [28]. They may occur along any segment of the urethra but are most commonly located proximally [29]. There should not be gross invasion of adjacent structures. At histology, urethral leiomyomas are noninvasive, slow-growing bundles of smooth muscle cells with some fibrosis. Cystic degeneration, hemorrhage and necrosis may also be present. Clinically, urethral leiomyoma may be palpable and may cause obstruction. Presentation can include dysuria, dyspareunia, hematuria, and recurrent urinary tract infection.
US and MRI may help to determine if the margins of suspected leiomyomas are well encapsulated or infiltrative. Leiomyomas tend to have well-encapsulated margins, while malignant lesions have infiltrative margins [30]. At US, a urethral leiomyoma appears as a solid, homogeneously isoechoic, well-circumscribed mass that demonstrates increased vascularity with Doppler [5]. On MRI, a typical urethral leiomyoma has intermediate signal intensity on T1-w images and low to high signal intensity on T2-w images and enhances homogeneously after intravenous gadolinium (Fig. 30.13).
Degenerating leiomyomas have variable signal intensity due to internal cystic components [31, 32] but are typically well circumscribed. Lesions that are poorly circumscribed with infiltrative margins become suspicious for malignancies and likely require tissue diagnosis for confirmation. Transformation to malignancy for leiomyomas has not been reported to our knowledge. MRI aids surgical planning because it delineates the anatomic relationships between the lesion and the surrounding structures. Treatment is with local surgical excision to alleviate mass effects and symptoms although these symptoms may recur if surgical resection is incomplete.
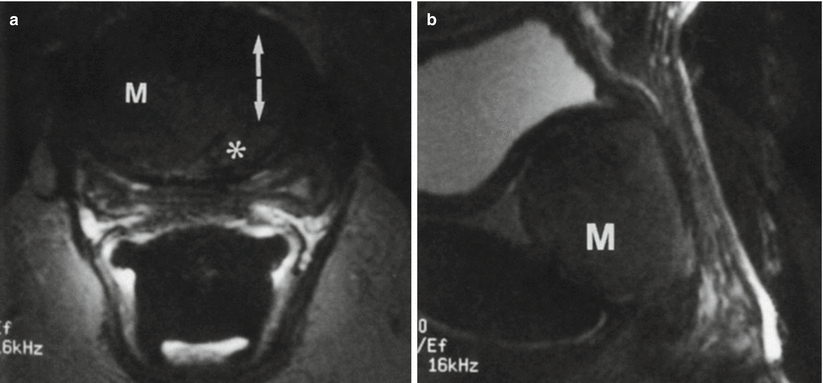

Fig. 30.13
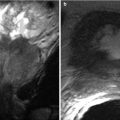
Urethral leiomyoma in a 54-year-old woman with a suspected vaginal mass. Axial (a) and sagittal (b) fast SE T2-w MR images obtained with an endorectal coil demonstrate a homogeneous low-signal-intensity mass (M) anterior to the vagina. The mass is located between the muscle bundles of the urethra (arrows in a), and the urethral lumen (*) is displaced to the left. Results of pathologic analysis confirmed a benign urethral leiomyoma (Used with permission from [16])
Urethral Duplication
Urethral duplication is a congenital anomaly occurring as a result of abnormal genitourinary development. Urethral duplication is more common in men than in women. The duplication may be complete, with two externally draining orifices, or, more commonly, incomplete, with one draining orifice and a second blind-ending tract that can be a nidus for stone formation or infection. In most cases, the more ventral urethra is the more normal of the two in terms of caliber and external orifice position [33]. In women, urethral duplication is often associated with other genitourinary anomalies including bladder duplication, ureteral duplication, ureteral ectopia, and renal agenesis [34, 35]. The typical initial clinical presentation occurs in the pediatric population. Symptoms include double urinary streams, incontinence, recurrent urinary tract infections, or obstructive voiding symptoms [36]. Surgical closure of the abnormal duplicated urethra, sometimes with resection of the intervening septal tissues, is the most common treatment.
VCUG can be used initially in patients with two external urethral orifices to demonstrate presence of duplicated urethras. This modality may, however, fail to demonstrate incomplete duplicated urethra in which the second tract does not have an external orifice. US demonstrates two anechoic urethral tracts originating from the bladder trigone or neck and extending to the perineum. However, if the urethras are not urine-filled, they may not be visible by US. On T2-w MRI, two hyperintense linear tracts originating at the bladder are detected. MRI can be helpful to evaluate incomplete urethral duplications that may not otherwise be visible on VCUG and to better define origin and termination of the tracts and their relationship to surrounding structures in order to differentiate this diagnostic entity from an ectopic ureter, a vesicourethral fistula, or a vesicovaginal fistula.
Urethral Fistula
Urethral fistulas are abnormal communications between the urethral lumen and other adjacent structures. They are classified based on the structure with which they communicate. They most commonly include urethrovaginal, vesicourethral, urethrorectal, and urethroperineal/urethrocutaneous subtypes [37]. Complex subtypes can involve multiple communications. The causes of urethrovaginal fistulas include GI diseases such as Crohn’s disease, systemic diseases such as Behçet disease, GU tract diseases such as bladder or urethral malignancies or stones, and iatrogenic complications including pelvic radiation therapy, postsurgical complications, and obstetric complications [38]. Vesicourethral fistulas are most commonly caused by urethral trauma or as a surgical complication. Urethrorectal fistulas are commonly congenital with associated complex anorectal developmental abnormalities [39] but, in adults, may also be caused by urethral instrumentation or radiation therapy [40, 41]. Urethroperineal and urethrocutaneous fistulas are acquired fistulas in patients with chronic decubitus ulcers or abscesses in the perineal region or urethra [42]. Clinical presentation is dependent on the type of fistula and may include incontinence of urine through the vagina or rectum, fecaluria, pneumaturia, recurrent urinary tract infections, and pain or irritation.
MRI enables noninvasive imaging of fistulas in multiple planes and defines the fistula tract’s relationship to adjacent soft tissue and vascular structures [43] for surgical planning. Contrast or fluid can be injected into the urethra to confirm exact communication routes and to better delineate complex fistula tracts. Although US and delayed contrast-enhanced CT can demonstrate larger fistula tracts, T2-w and gadolinium-enhanced fat-saturated T1-w MRI enable detection of small and complex fistulas. Urethral fistulas manifest as T2-w hyperintense tracks extending from the urethra to the vagina, urinary bladder, rectum, or perineum (Fig. 30.14). While direct visualization of the fistulous tract at MRI may not always be possible, secondary signs such as focal enhancement from inflammation with loss of intervening fat planes can be helpful [6] and injection with dilute gadolinium can improve detection and visualization.
Diversion of urine via nephrostomy or stents and surgical repair of the fistula tract are the mainstay of management, although success rates vary with the type of fistula and the state of surrounding tissue. For example, adjacent irradiated tissue or extensive neoplasms have lower rates of surgical success, and palliative measures may be the predominant treatment option [44].
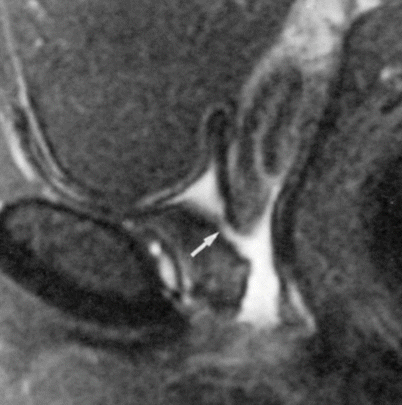

Fig. 30.14




Urethrovaginal fistula in a 23-year-old woman with a history of prior vaginal septum resection. Sagittal fat-suppressed T2-w MR image obtained with a pelvic coil demonstrates a fistula (arrow) between the proximal urethra and the vagina. The bladder is empty (Used with permission from [16])
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree