(1)
Department of Radiology, Columbia University Medical Center, 180 Fort Washington Avenue, New York, NY 10032, USA
(2)
Department of Surgery, Guthrie Robert Packer Hospital, 114 W. Packer Ave, Sayre, PA 18840, USA
(3)
Department of Diagnostic Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Keywords
SpleenComputed tomographyMagnetic resonance imagingAnatomy
Most of the spleen lies in the intraperitoneal cavity. The notable exception is the splenic hilum. The splenorenal ligament invests the tail of the pancreas and envelops the splenic vessels at the splenic hilum. The splenic hilum therefore lies in the retroperitoneal space. Knowledge of both spaces is important because splenic trauma can result in both intraperitoneal and retroperitoneal hematomas. The spleen is attached to the surrounding structures by the splenorenal, gastrosplenic, and phrenicolic ligaments [1]. The gastrosplenic and phrenicolic ligaments are intraperitoneal. The splenorenal ligament is retroperitoneal.
The spleen has a smooth serosal surface and is attached to the retroperitoneum by fatty ligaments that also contain its vascular supply. The splenic surfaces are described relative to their locations and are termed the diaphragmatic (phrenic) and visceral surfaces. The visceral surface is divided into an anterior or gastric ridge and a posterior or renal portion. The splenic hilum is directed anteromedially.
The spleen is supplied by a splenic artery, which is a branch of the celiac axis, and drained by a splenic vein that passes directly posterior to the pancreas. The splenic artery and vein emerge from the splenic hilum in the form of six or more branches. The artery, which is slightly superior to the vein (Fig. 8.1), is remarkable for its large size and tortuosity. The short gastric, gastroepiploic, pancreatic, and inferior mesenteric veins drain into the splenic vein. The splenic and superior mesenteric veins join at the portosplenic confluence to form the portal vein.
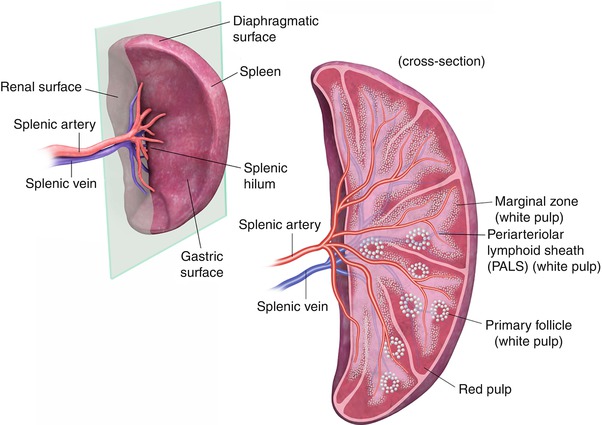

Fig. 8.1
The splenic surfaces are described relative to their locations and are termed the diaphragmatic (phrenic) and visceral surfaces. The visceral surface is divided into an anterior or gastric portion and a posterior or renal portion. The splenic hilum is directed anteromedially. The splenic artery and vein emerge from the hilum in the form of six or more branches; the splenic artery is slightly superior to the vein
At the microscopic level, the spleen is divided into two compartments, the red and white pulps. These compartments are separated by the marginal zone. The white pulp is made up of T and B lymphocytes and is located centrally. The red pulp is composed of rich plexuses of tortuous venous sinuses.
Embryology
The spleen is derived from dorsal mesenchymal condensations that, by the end of the fifth week of human gestation, begin to differentiate into the spleen. During embryogenesis, as the spleen rotates into position (Fig. 8.2) in the left upper quadrant of the abdominal cavity, a connection, called the splenorenal ligament, is formed between the left kidney and the spleen. This ligament also invests the tail of the pancreas. The rotation places the splenic hilum and vessels in the retroperitoneum; the rest of the organ is intraperitoneal. As the spleen rotates, the mesenchymal condensations can remain in various connecting ligaments, leading to splenules and sometimes intrapancreatic rests.
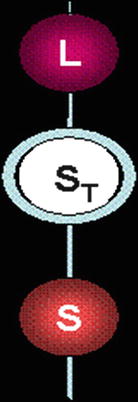

Fig. 8.2
During embryogenesis, rotation of the spleen places it in the left upper quadrant of the abdomen. L Liver, ST Stomach, S Spleen
Techniques
Computed Tomography (CT)
Intravenous contrast should be used to image the spleen. Multiphase imaging is appropriate in most settings. Single-phase imaging should be performed in the portal venous phase because the parenchyma demonstrates an arcuate enhancement pattern on arterial-phase imaging, making it difficult to assess splenic lesions (Fig. 8.3). If the primary concern is for vascular disease, early arterial imaging can be used. Oral contrast is not necessary in the trauma setting; indeed, the delay involved and the possible obscuration of blood products explain why oral contrast may not be used. In the nonemergency setting, oral contrast may help delineate primary splenic disease from adjacent gastric, small bowel, or colonic disease.
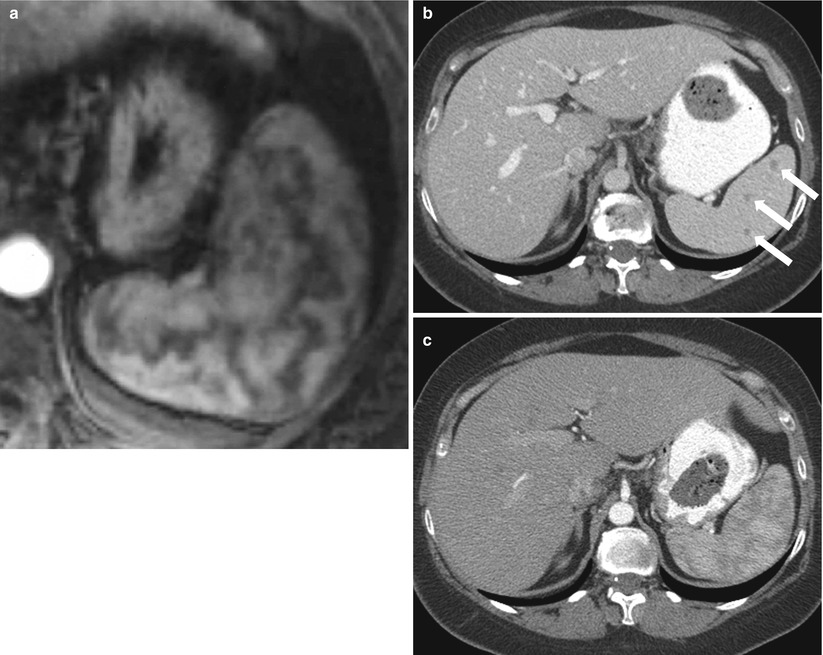

Fig. 8.3
Arciform Enhancement Pattern. Axial contrast enhanced T1-weighted in arterial phase (a) exhibits a heterogeneous enhancement of the spleen “arciform pattern” that may be obscuring lesions. Axial contrast CT images obtained in venous (b) and arterial phases (c) in a different patient reveal several tiny lesions in venous phase (arrows in b) that are obscured in CT images obtained in arterial phase (c)
Magnetic Resonance (MR) Imaging
MRI has the advantages of providing multiplanar, multi-sequence images and not requiring radiation. When dedicated imaging of the spleen is required, routine sequences are sufficient to evaluate the liver. The normal adult spleen is isointense to muscle on T1-weighted images and hyperintense to the liver on T2-weighted images. Studies have demonstrated a lower T2 signal in neonate spleens; this should not be misinterpreted as a pathological abnormality [2].
Various pathological entities can be illustrated in Table 8.1.
Table 8.1
Pathological classification of splenic disease
Congenital | Inflammatoryor infectious | Vascularlesions | Trauma | Benign neoplasm | Malignantneoplasm | Splenomegaly(cause of) |
|---|---|---|---|---|---|---|
Polyspleniaor asplenia | Bacterial orpyogenic abscess | Splenic arteryaneurysm | Grade I | Hemangioma orhemangiomatosis | Angiosarcoma | Portalhypertension |
Heterotaxy | Grade II | Hamartoma | Metastases | Lymphoma | ||
Congenitalcyst | Fungal | Splenic veinthrombosis | Grade III | Lymphangioma | Lymphoma | Leukemia |
Splenule orchoristoma | Mycobacterial | Infarction | Grade IV | Littoral cellangioma | Other rare neoplasms | Anemia or irondeposition |
Splenic cleft | Parasitic: mainlyechinococcosis | Varices orsplenorenal shunt | Grade V | Other rareneoplasms | Storage diseases | |
Wanderingspleen | Viral: mainlyEpstein-Barr | Gamna-Gandybodies | Post-traumatic cyst | Congestiveheart failure | ||
Infectiousmononucleosis | ||||||
Heterotopicsplenic tissue | Inflammatorypseudotumor | Splenosis | Amyloidosis andother rare causes |
Algorithmic Approach to Splenic Lesions
Evaluation of the spleen should include the size, number, and location of the spleen and splenic bodies. The perfusion, density, and presence of mass lesions should also be assessed (Algorithm. 8.1).
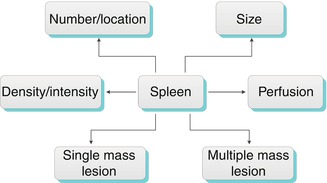

Algorithm 8.1
Algorithm for evaluation of the spleen
Important information can be gleaned from the initial impressions of the spleen. Is it in the normal location? Is it normal in size? Are there multiple splenic bodies and, if so, where are they located? These seemingly simple questions allow the differential diagnosis of an unusual-appearing spleen to be narrowed down.
Accessory Spleens
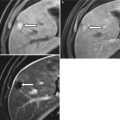
Also referred to as splenules, accessory spleens occur in 30–40 % of patients. Accessory spleens are usually multifocal and most often are located in the hilum, but they can occur anywhere around the spleen [3] and, rarely, in the pancreatic tail (also termed splenic choristoma, discussed in the pancreas chapter). Splenules follow normal splenic CT and MR imaging attenuation and signaling on all phases and sequences (Fig. 8.4). When there is doubt as to whether a mass lesion represents a splenule, sulfur colloid scans can provide additional diagnostic information, because the radiolabeled particles are taken up by the splenules (as well as the spleen).
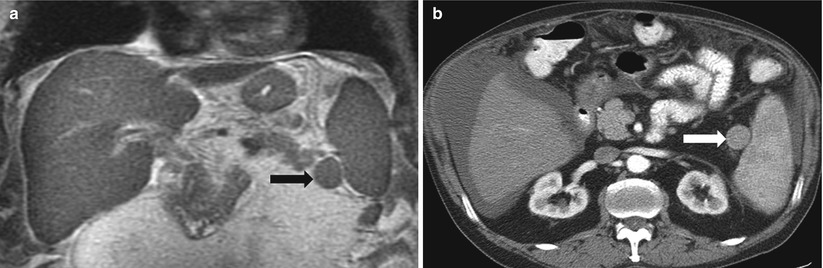

Fig. 8.4
Accessory spleen. Coronal single-shot fast spin-echo T2-weighted (a) and axial arterial-phase contrast-enhanced CT image (b) demonstrate a small rounded mass (arrow) in the splenic hilum that has imaging features identical to those of the spleen, compatible with an accessory spleen
If surgical removal of the spleen is necessary, it is important to be aware of any accessory spleens, because these may need to be removed as well. Conversely, in the setting of traumatic splenectomy, preserving the splenules may allow splenic function to be maintained. Accessory spleens should not be overlooked during imaging interpretation because they can be affected by conditions similar to those involving the spleen (Fig. 8.5).
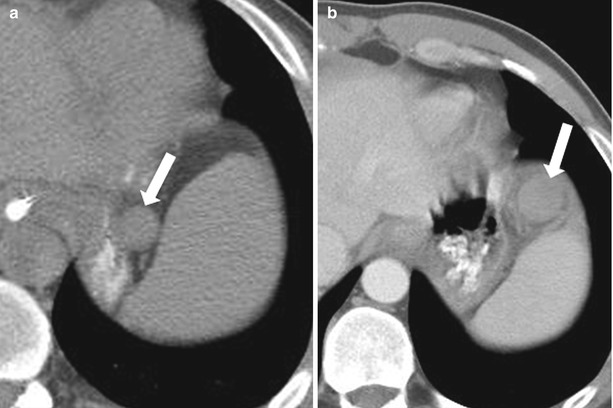

Fig. 8.5
Infarcted splenule. Nonenhanced CT (a) and axial contrast enhanced CT (b) (b performed 2 months after a). Compared with (a), (b) reveals a more anterior location of the splenule (arrow), with development of generalized hypoperfusion, and surrounding stranding, consistent with infarcted splenule
Splenic Cleft
As mesenchymal components fuse, incomplete fusion can appear as splenic clefts or lobulations (Fig. 8.6). Although a normal splenic variant, it can be confused with traumatic injury.
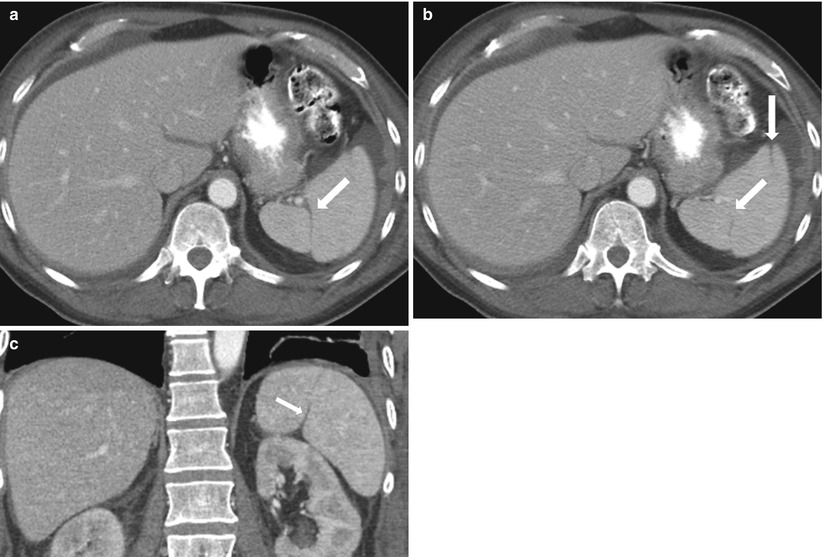

Fig. 8.6
Splenic clefts. Axial contrast-enhanced CT (a, b) and coronal reformatted (c) images demonstrate splenic clefts and lobulations (arrows) in this patient with a history of gastrointestinal stromal tumor and no history of splenic trauma
Key Teaching Point; Abnormal Location of the Spleen. Heterotaxy syndrome (reversed, also noted in polysplenia), wandering spleen, heterotopic splenic tissue, intrapancreatic splenule (mimics hypervascular mass in the pancreatic tail).
Heterotaxy Syndromes
Like other viscera, the spleen is involved in situs abnormalities. When the viscera are in their expected locations, this is referred as situs solitus; a complete mirror-image rearrangement is called situs inversus. Situs ambiguous applies when some organs are in their expected locations and others are not. If the purpose of a radiological test is to evaluate and characterize situs anomalies, attention should be paid to the location of the heart chambers, which is beyond the scope of the chapter. There is a significant overlap of findings between heterotaxy and polysplenia or asplenia [4, 5].
Wandering Spleen
The spleen is attached to surrounding structures by splenorenal, gastrosplenic, and phrenocolic ligaments. If these ligaments are absent, the spleen is no longer anchored in position and can “wander” through the abdominal cavity (Fig. 8.7). Such displacement can lead to twisting on the vascular pedicle, causing infarction [6].
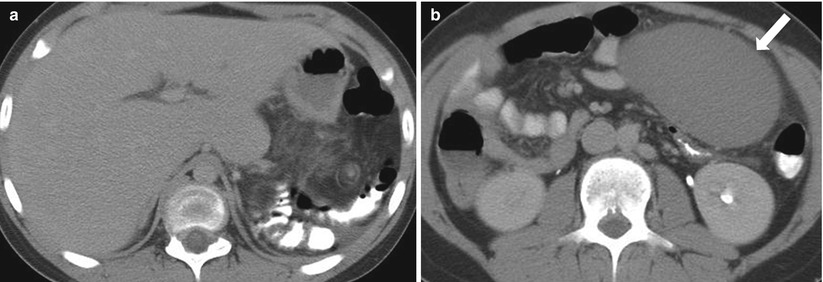

Fig. 8.7
Wandering Spleen. Axial nonenhanced CT images from the same patient at different levels. The spleen is absent in the left upper quadrant (a) and has located to the left lower quadrant (arrow in b) and exhibits less attenuation than expected
Heterotopic Splenic Tissue
A splenogonadal fusion is an anomaly characterized by the presence of splenic tissue in the testis, epididymis, and spermatic cord [7]. A few rare cases of ovarian fusion have been reported as well [8]. Splenorenal fusion is another rare anomaly that is characterized by the presence of splenic tissue in the renal parenchyma [9, 10].
Intrapancreatic Splenule
As discussed in the pancreas chapter, intrapancreatic splenules are typically located in the pancreatic tail and can mimic hypervascular pancreatic masses.
Abnormal number of splenic bodies: Absent/asplenia, increased number/polysplenia (congenital) associated with other congenital defects, splenosis (traumatic), accessory spleens (most common location in hilum) (Algorithm. 8.2)
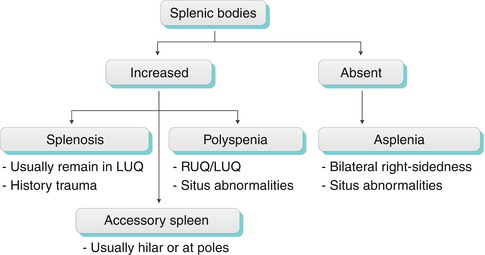

Algorithm 8.2
Algorithm for interpreting the number of splenic bodies. LUQ left upper quadrant, RUQ right upper quadrant
Polysplenia
Polysplenia is characterized by a complex set of congenital anomalies, including multiple rounded lesions with typical features of splenic tissue (Fig. 8.8), bilateral bilobed lungs, hyparterial bronchi, a midline or left-sided liver, and a variable stomach location [11]. An association between polysplenia, congenital lobar emphysema and azygos continuation of the inferior vena cava has been reported [12]. The splenic bodies lie along the greater curvature of the stomach and appear as multiple similar appearing lesions that measure 1–6 cm [13]. Without knowledge of congenital anomalies, this can be difficult to differentiate from splenosis in the setting of prior trauma (Fig. 8.8).
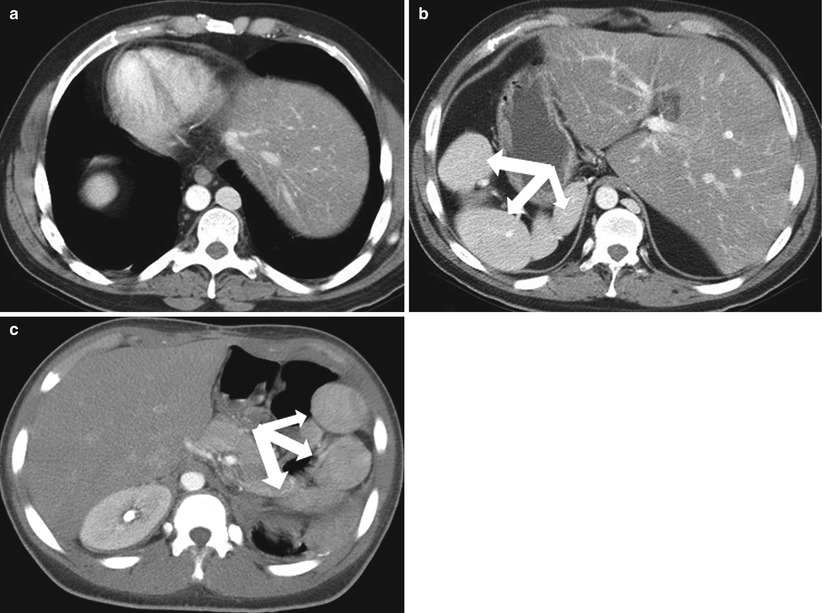

Fig. 8.8
Polysplenia. Axial contrast enhanced CT images from the same patient demonstrate dextrocardia (a) and a left-sided liver (b). Multiple rounded masses (arrows) with typical features of splenic tissue in the right upper quadrant are compatible with polysplenia. (c) Contrast-enhanced CT image in a different patient reveals multiple rounded splenic bodies consistent with polysplenia in situs solitus
Splenosis
Splenosis is the presence of viable splenic tissues in other anatomical compartments of the body that occur secondarily to traumatic or iatrogenic rupture of the spleen. Splenosis can be differentiated from accessory splenules on the basis of the patient’s history and the location and number of lesions (Fig. 8.9). Hypertrophied splenic components in splenosis are usually large and are not found in the typical location for splenules (i.e., in the splenic hilum). Hypertrophied components can exist in either intraperitoneal or extraperitoneal locations compatible with the splenic hilum lying in the retroperitoneal space and the rest of the spleen lying in the intraperitoneal space (Fig. 8.9).
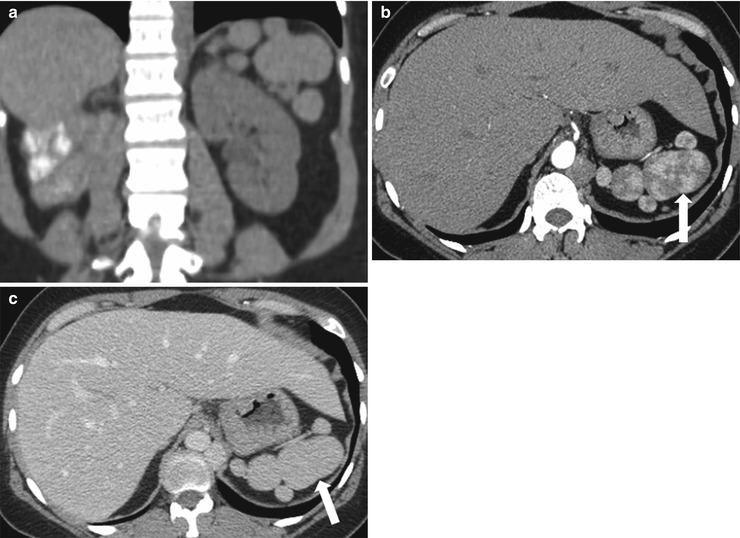

Fig. 8.9
Splenosis. Coronal reformatted image of contrast enhanced CT (a), axial contrast enhanced CT images in arterial (b) and venous (c) phases demonstrate multiple lesions (arrows) with serpentine enhancement pattern in the arterial phase which become homogeneous in the portal venous phase
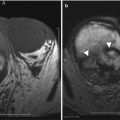
Asplenia
Asplenia refers to an absent spleen. With this condition, the liver is usually midline, the inferior vena cava is left-sided, and the stomach is often absent [4] (Fig. 8.10). Associated cardiac anomalies are complex and severe and carry 80 % mortality rate for the first year of life [4].
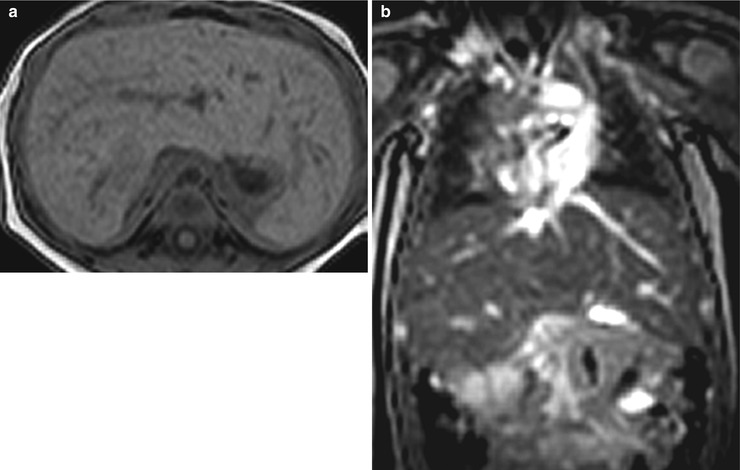

Fig. 8.10
Asplenia. Axial T1-weighted (a) and coronal T2-weighted (b) MR images of a midline liver and an absent spleen. The patient had complex congenital disease, including dextrocardia and total anomalous pulmonary venous return
The splenic cleft can be noted as a variant and should not be mistaken for a laceration. An increased number of splenic bodies can be seen with accessory spleens, polysplenia (congenital complex anomaly), and splenosis (post-traumatic). On the other hand, the spleen can be absent (asplenia). An abnormal location may be noted with wandering spleen. Rarely, ectopic splenic tissue is seen in other tissues, as discussed above.
Interpretation of Splenic Focal Lesions
Splenic lesions are best interpreted via an algorithmic approach. This approach, along with the appropriate clinical context, will help narrow the differential diagnosis. A single disease can manifest in various patterns; both common and uncommon presentations are discussed here. Care should be taken to interpret the spleen in the portal venous phase of imaging because the arcuate enhancement pattern in the arterial phase makes it difficult to distinguish artifacts from true lesions.
The approach begins with determining the number of lesions and their density or intensity.
Key Teaching Points; Cyst-like lesionsCyst-like lesions should have low density on CT images (0–20 HU), low signal intensity on T1-weighted images, and high signal intensity (such as simple fluid) on T2-weighted images.
The term apparently cystic is used because they can be of low attenuation and too small to characterize by CT. They are also included in the differential diagnosis of multiple apparently solid lesions.
These diagnoses can be single or multiple, according to the pathological type.
Simple cystic-looking splenic lesion: Primary cyst (congenital), secondary (post-traumatic/inflammatory), abscess (symptomatic), lymphangioma (very rare, asymptomatic).
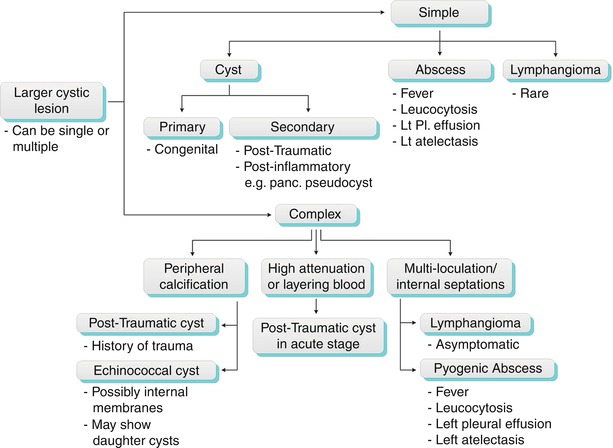
The first step in the diagnostic approach to a focal splenic lesion (or lesions) is to determine whether it is cystic or solid. Numerous small cystic lesions may be too small to characterize on CT and will appear indeterminate. The main differential for these indeterminate lesions includes fungal and mycobacterial abscesses and, rarely, multiple small cysts (Algorithm. 8.3). Sarcoid lesions are not cystic, but they can be included in the differential diagnosis due to their being too small to characterize. A larger, simple lesion can represent a congenital cyst, a pyogenic abscess, or in rare cases lymphangioma. A complex cystic lesion can represent an acute post-traumatic cyst (containing blood product), a peripherally calcified cyst (old post-traumatic cyst or echinococcal hydatid cyst), or a septated cyst (such as in lymphangioma and abscesses) (Algorithm. 8.4).
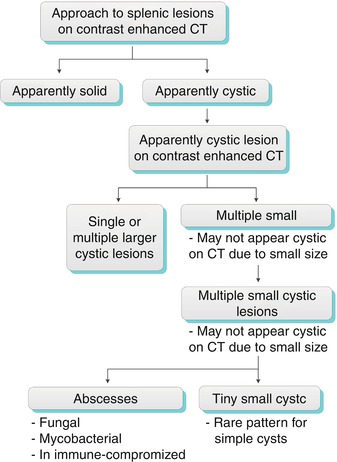

Algorithm 8.3
Algorithms for practical diagnostic approach of splenic cystic lesions

Algorithm 8.4
Algorithm for practical diagnostic approach of larger splenic cystic lesions
Splenic Cysts
Congenital splenic cysts have an endothelial lining and are therefore true cysts [15]. On CT images, they appear homogeneous and well-circumscribed and have a thin or almost imperceptible wall. Internal septations may be present. On precontrast imaging, cysts have a density of less than 20 HU and no enhancement. Lesions are typically markedly hyperintense on T2-weighted MR images and homogeneously hypointense, without enhancing components, on T1-weighted MR image (Fig. 8.11). If symptomatic, these lesions can be treated by cystectomy [14, 16].
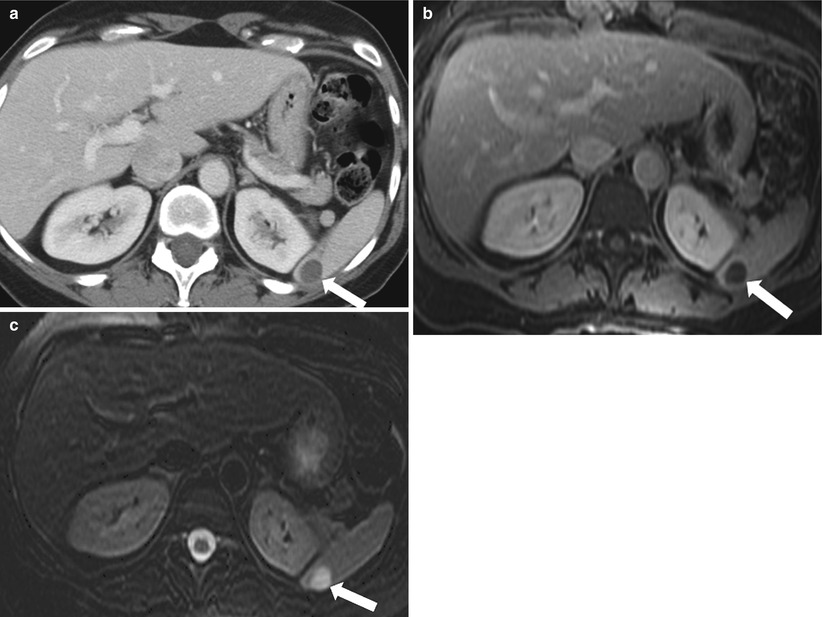

Fig. 8.11
Simple Cyst. Axial contrast enhanced CT (a), contrast enhanced T1-weighted (b), and fat-saturated T2-weighted (c) images of the upper abdomen reveal a well-circumscribed, fluid-attenuated, unenhanced lesion (arrow). The lesion demonstrates low signal intensity with no enhancement and high signal intensity on T2-weighted image (arrow), compatible with a simple cyst
Pyogenic Abscess
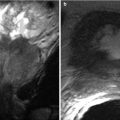
Pyogenic abscess is usually secondary to hematogenous spread. Risk factors include intravenous drug abuse, trauma, endocarditis, systemic infection, and immunodeficiency [17]. Direct inoculation in the setting of trauma has also been described. On CT images, these abscesses exhibit low attenuation, whereas on MR images, they demonstrate central T2 hyperintensity and low T1 signal intensity (Fig. 8.12). A rim-like enhancement may be noted. The presence of gas is consistent with a diagnosis of abscess formation. Pyogenic abscesses also demonstrate restricted diffusion, although this is not specific to the condition. Splenic abscesses should be suspected in febrile patients with leukocytosis, left upper quadrant pain, and left-sided pleural effusion [18]. Splenectomy and antibiotics have been used to treat these lesions [19, 20]. Abscesses <4 cm have been successfully treated with antibiotics only; a favorable outcome for larger lesions has been reached with percutaneous drainage and intravenous antibiotics [21].
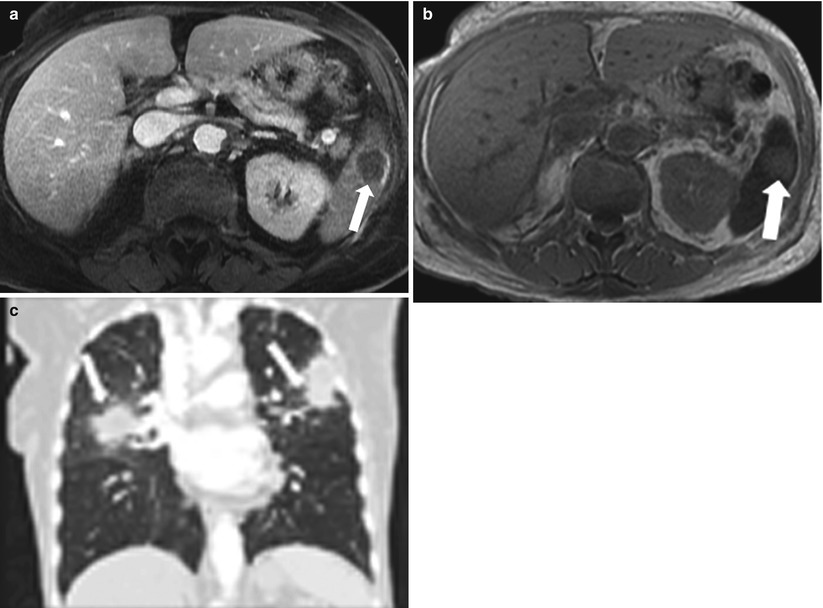

Fig. 8.12
Pyogenic Abscess. Axial contrast enhanced T1-weighted (a) and axial T2-weighted (b) MR images demonstrate a well-circumscribed lesion exhibiting a low signal intensity with a rim enhancement (a) and high signal intensity on T2-weighted (b), representing abscess. Coronal reformatted image of the chest reveals bilateral airspace opacities most compatible with pneumonic consolidation
Lymphangioma
Because lymphangiomas are composed of dilated lymphatic ducts, the contents are chylous. They appear as non-enhancing cystic structures with lobulated contours. Thin internal septations can also be seen. Calcification and internal debris are absent. Lymphangiomas are sharply demarcated, with no enhancement on post-contrast imaging (Fig. 8.13). Multiple lymphangiomas can be seen in the setting of lymphangiomatosis [22].
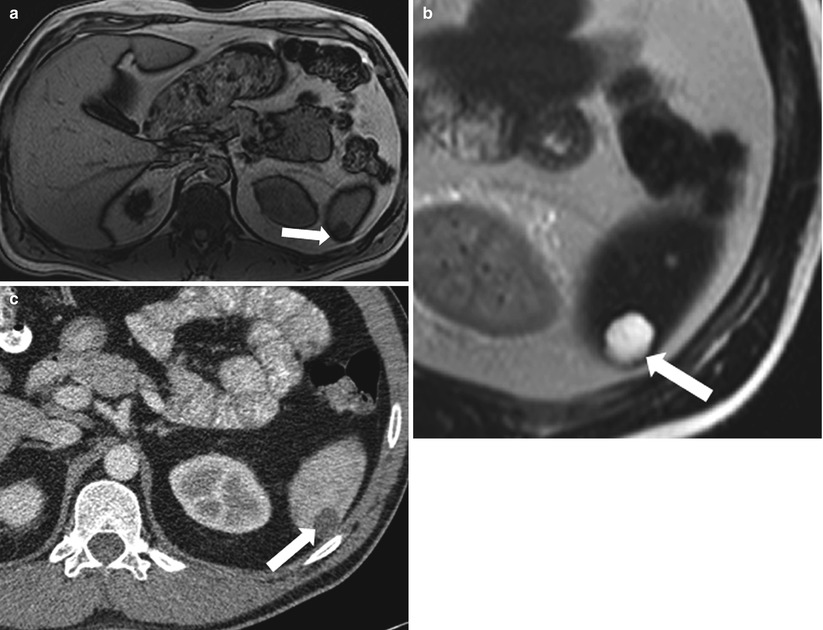

Fig. 8.13
Lymphangioma. Axial in-phase T1-weighted (a), non-fat-saturated T2-weighted (b) and contrast-enhanced axial CT image (c) demonstrate a cystic lesion (arrow) in the spleen exhibiting low signal intensity on T1-weighted and high signal intensity on T2-weighted images. Axial contrast enhanced CT (c) shows a lobulated border. These findings are suggestive of lymphangioma
Key Teaching Points; Multiple small cystic looking lesionsSome low attenuation lesions (such as sarcoidosis) can be misinterpreted as cystic lesions on CT as they can be too small to characterize.
If multiple small low attenuation lesions are noted the main differential diagnosis includes Sarcoidosis, fungal/mycobacterial infections, or tiny cysts (rare).
Fungal and Mycobacterial Abscesses
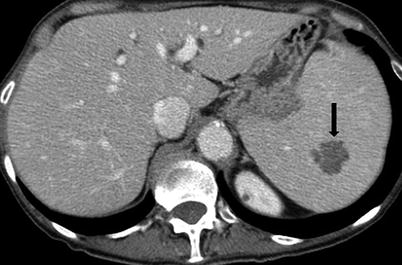
Multiple fungal microabscesses in the spleen usually occur in immunocompromised patients. In such cases, Candida is the most common fungus, followed by Aspergillus and Cryptococcus. Mycobacterial abscesses can be caused by Mycobacterium tuberculosis, Salmonella spp., Pneumocystis jiroveci, and Mycobacterium avium-intracellulare. Microabscesses appear on CT images as multiple small hypodense lesions less than 2 cm in the longest dimension, usually ranging from 2 to 5 mm (Fig. 8.14). On MR images, these lesions are isointense on T1-weighted images and mildly hyperintense on T2-weighted images. Peripheral enhancement can be difficult to appreciate on CT and MR images. Treatment and diagnosis of the abscesses depend on the etiological agent, environmental risk factors, and cause of immunodeficiency.
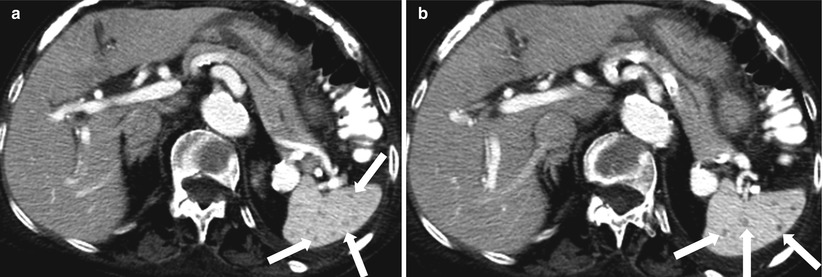

Fig. 8.14
Fungal abscesses. Axial contrast enhanced CT (a, b) images reveal multiple hypodense lesions (arrows) measuring 1–2 mm throughout the spleen. These lesions represented fungal abscesses in immunocompromised patient and resolved after appropriate antifungal treatment
Key Teaching Points; Complex cystic lesionsThese are cystic lesions which have some complex features such as septations, blood, calcifications and enhancement. Diagnostic possibilities include hemorrhagic, acute traumatic cyst; calcified, post-traumatic cyst, echinococcosic; multilobulated (with internal septations), lymphangioma, and abscess.
Post-traumatic Cyst
Secondary cysts in the spleen are often traumatic [23, 24]. In the acute and subacute stages, layering blood products can be seen. Acute cysts may contain fluid with higher attenuation than simple fluid does. Variable signal intensities of blood degradation products can be seen on MR images (Fig. 8.15). Peripheral calcification is not seen in the acute setting. On MR images, acute traumatic cysts have an increased signal on T1-weighted images.
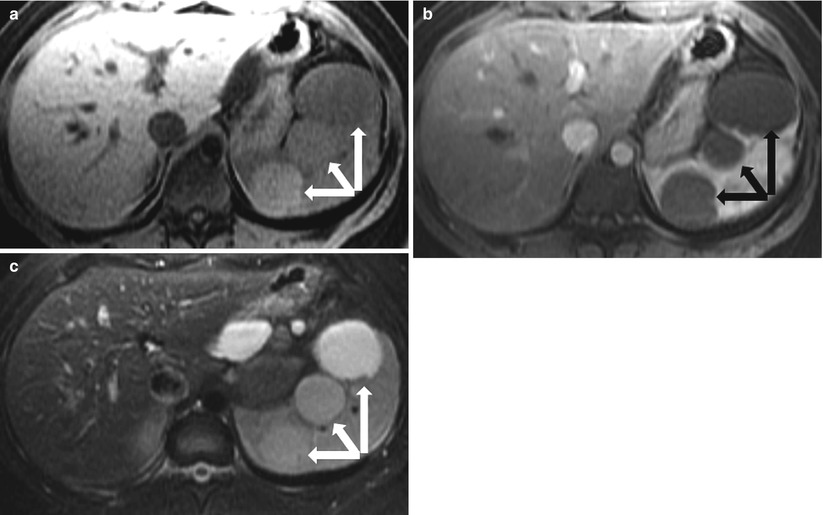

Fig. 8.15
Post-traumatic cysts in subacute stage. Axial precontrast T1-weighted (a), axial contrast enhanced T1-weighted (b), and fat-saturated T2-weighted (c) images in a patient with recent trauma demonstrate multiple well circumscribed lesions (arrows) exhibiting high signal intensity on T1-weighted (a) and T2-weighted (c) images, with no significant postcontrast enhancement, most compatible with post-traumatic cysts with signal intensities similar to those of blood products in subacute stage
A long-standing post-traumatic cyst has a homogeneously increased T2 signal and low T1 signal intensity. The rim has a low signal on all sequences, with evidence of blooming on gradient echo (GRE) sequences. On CT images, chronic post-traumatic cysts commonly demonstrate central low density and have a peripheral calcified rim (Fig. 8.16). Internal calcifications and septations are usually absent. Treatment is indicated if the cysts are symptomatic or at high risk of rupture (>5 cm) [25]. Both splenectomy and laparoscopic cystectomy can be performed [26]. Cystectomy preserves residual splenic tissue, allowing certain immune-based functions to be maintained [27]. Percutaneous drainage has not been found to be successful in treating these cases [28].
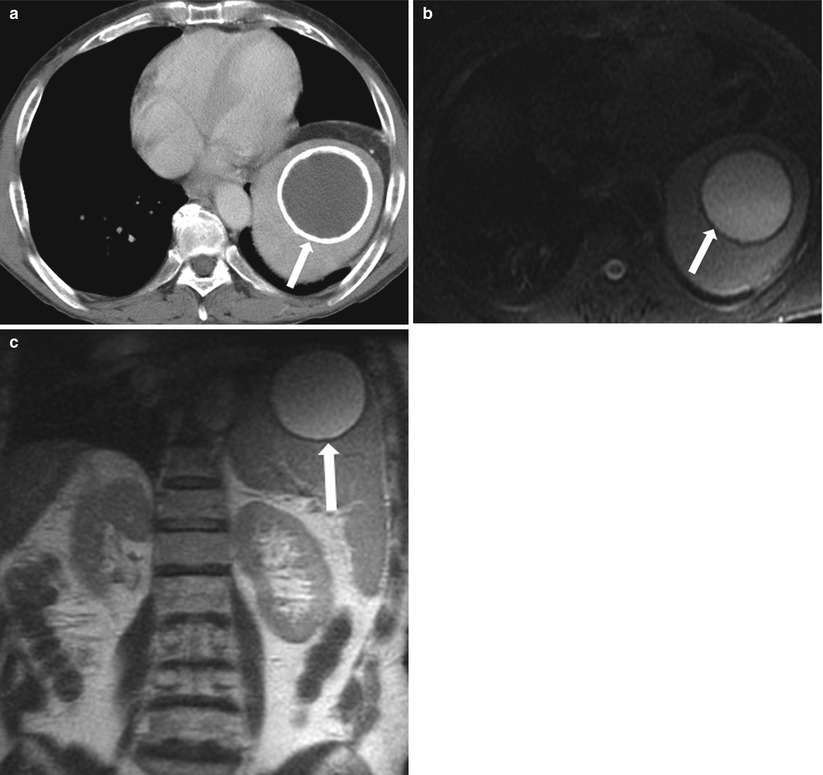

Fig. 8.16
Longstanding post-traumatic cyst. Axial contrast-enhanced CT (a) demonstrates a well-circumscribed, peripherally calcified splenic cystic lesion (arrow), with homogeneous internal low-density fluid present in this cystic structure. Axial fat-saturated T2-weighted (b), and coronal T2-weighted (c) images demonstrate the cystic lesion demonstrating internal fluid with a low signal intensity on T1-weighted and high signal intensity on T2-weighted images with peripheral curvilinear calcification (arrow) demonstrating low signal intensity on T1 and T2-weighted images. Follow-up, and stability of this lesion over at least 8 years. Findings are consistent with a longstanding post-traumatic cyst
Echinococcal Cysts
The liver is the most common organ involved in echinococcosis, which is most commonly caused by Echinococcus granulosus and less commonly by E. multilocularis. Early echinococcal cysts (also called hydatid cysts) may appear simple on CT images, with central low density, or they may have a high T2 signal on MR images. Unlike simple cysts, echinococcal cysts often have a perceptible wall that exhibits low signal intensity on T2-weighted images. The presence of floating membranes (intracystic linear or serpiginous low T2 signal structures) or daughter cysts (intracystic small cysts) is diagnostic. MR imaging allows for better demonstration of the cyst and layering membranes. Wall calcification may be also present and is thought to be correlated with disease activity; calcified cysts may indicate inactive disease [29] (Fig. 8.17). Current treatment strategies include splenectomy or, if possible, partial cyst excision [30]. Sclerotherapy has also been used successfully, especially for smaller cysts (<5 cm) [31].
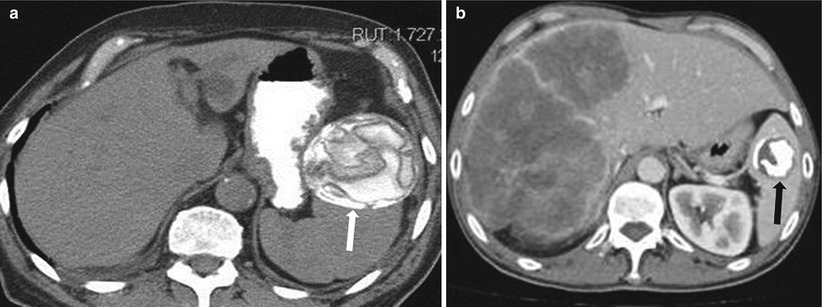

Fig. 8.17
Calcified hydatid cyst. Axial nonenhanced CT (a) and axial contrast enhanced CT in a different patient (b) demonstrate hydatid cysts in two patients; calcific lesion with floating membranes (arrow in a) and densely calcified hydatid cyst (arrow in b)
Multilobulated Cystic Lesions
Multilobulated cystic lesions include pyogenic abscesses and lymphangioma (Fig. 8.18), which were discussed above.