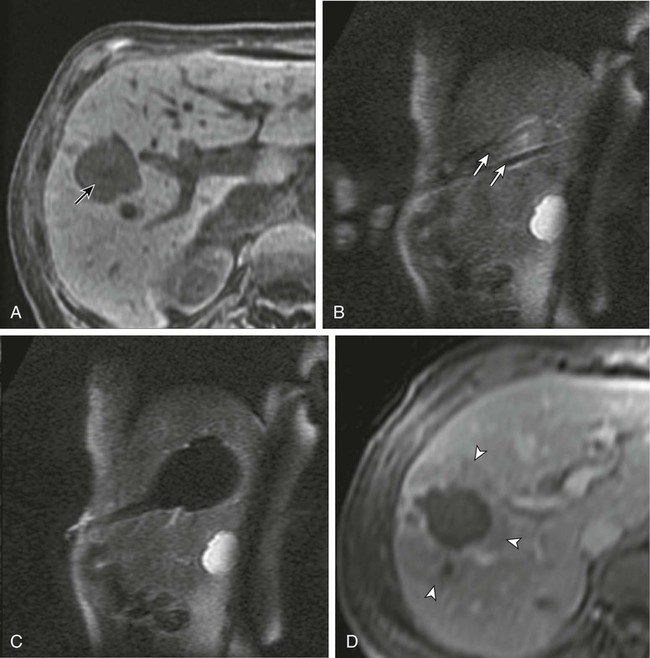
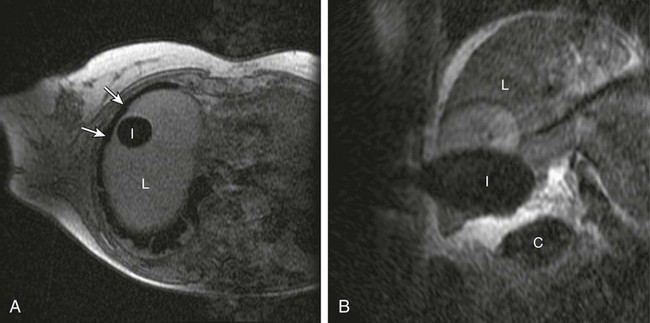
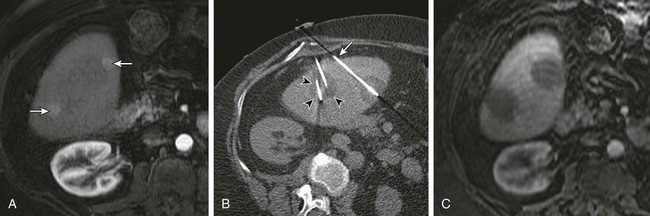
Chapter 143 Mark D. Mamlouk, Eric vanSonnenberg, Stuart G. Silverman, Paul R. Morrison, Charles D. Crum and Kemal Tuncali With the development and maturity of ablation techniques, radiologists now can treat a wide variety of solid tumors. There are several options when deciding which technique to use. Radiofrequency, microwave, laser, ethanol, and cryoablation all have been shown to destroy tumor tissue effectively.1–5 Although the choice of ablation strategy is usually based on institutional expertise and availability, these techniques (and other nonradiologic methods—i.e., surgery, chemotherapy, and radiation therapy) occasionally can be combined for a given patient, depending on the location and specifics of the target. This chapter focuses on the use of cryoablation to treat neoplasms that involve the liver. For several decades, cryoablation has been used surgically to treat malignancies.6 It was first described in the liver in 1963.7 Because of the large size and assorted geometries of the probes, the surgeon would place them from an open or laparoscopic approach with the aid of intraoperative ultrasound. However, the development of thin needle-like probes and the use of computed tomography (CT) and magnetic resonance imaging (MRI) have allowed safe and effective use of percutaneous cryoablation.6 Currently, percutaneous cryoablation is used to treat a variety of primary neoplasms throughout the body, including those in the liver, kidney, lung, prostate gland, and musculoskeletal systems. The mechanism of tumor kill is multifactorial, but includes predominantly cell dehydration, cell membrane rupture, and ischemia, the latter due to vascular stasis and thrombosis.8,9 Cryoablation can be used to treat primary malignant liver lesions such as hepatocellular carcinoma (HCC), as well as benign lesions such as symptomatic hemangiomas and liver adenomas.10,11 Nonetheless, the most common use of ablation in the liver in the United States is for metastatic disease from colon, breast, and neuroendocrine primaries (Fig. 143-1). Because fewer than 15% of patients with metastatic liver tumors are candidates for curative surgical resection, percutaneous tumor ablation is a viable alternative. Likewise, a minority of patients with primary HCC are candidates for curative surgical resection. Tumors often are deemed surgically unresectable because too much liver parenchyma would be sacrificed, or the tumor lies too close to major biliary or vascular structures such that adequate margins cannot be obtained. Another restriction for surgery is various comorbid conditions, such as cardiopulmonary disease or nutritional status. A percutaneous approach allows treatment of these problematic tumors for surgery because the tissue destruction can be targeted precisely at the tumor and the immediately surrounding adjacent liver parenchyma, with preservation of a greater percentage of normal parenchyma.10 Likewise, cryoablation also may be able to induce tumor necrosis when vascular or biliary structures are challenging for the surgeon because of proximity of the tumor to vital structures in the hepatic hilum (although radiologists too must exercise great caution in these same anatomic regions). Cryoablation also has been used to treat primary liver neoplasms, most commonly HCC. Indications for cryoablation of primary HCC are different from those for metastatic disease because of the underlying pathology. HCC is caused by underlying chronic liver disease that is usually secondary to hepatitis or alcoholic liver disease. Because of the underlying disease, the only definitive treatment of HCC is liver transplantation. As a result of the limited availability of donated livers and the high cost of transplantation, only a very small percentage of patients can undergo this option. Surgery can offer complete resection of the first HCC and any satellite lesions, but a second or more lesions may have already developed or will develop in the overwhelming majority of patients. If surgical resection was chosen to treat the primary lesion, only a fraction will be able to undergo a second resection.12 Ablation affords the possibility of complete destruction of the first and subsequent lesions, without significant loss of functioning liver parenchyma. We have treated patients up to five times percutaneously for secondarily diagnosed lesions. Strategically, patients undergo surveillance, and as new lesions appear, they too can undergo ablation. Cryoablation also has been used to treat benign liver lesions, specifically hemangiomas and adenomas.10,11 Adenomas are seen predominantly in younger women taking oral contraceptives; the tumors occasionally cause abdominal pain. Cessation of oral contraceptives is first-line therapy, but with persistent problems (especially pain), more invasive treatment may be necessary. Cryoablation is a valuable alternative because of the amount of liver that can be preserved and the low risks involved in the ablation procedure. Symptomatic focal nodular hyperplasia can be treated as well. Sometimes patient positioning (i.e., prone versus supine) can provide adequate distance to avoid injury to critical structures. If positioning of the patient does not provide adequate safety of adjacent structures, sterile water, dextrose, or a normal saline barrier can be interposed (Fig. 143-2). This is accomplished percutaneously by injecting sterile fluid through a fine needle between the lesion and the bowel to be displaced, termed hydrodissection.13,14 Overall, when care is taken in both planning and execution of cryoablation, it is a relatively safe procedure. During the early days of cryosurgery, the probes were cooled with liquid nitrogen. Because these LN2 probes were relatively large (3- to 8-mm in outer diameter), for years cryoablation was practical only in a surgical setting.15 Because the size of the probe was less than optimal, a complicated Seldinger technique was used that required several minutes to position and reposition the probes. Even though these probes were effective in causing tissue destruction, the probe size and handling of the liquid nitrogen were negative features in light of the now contemporary gas-based systems. The argon gas–based systems that were developed in the 1990s have virtually replaced liquid nitrogen devices. Argon systems work on the Joule-Thompson principle, in which low temperatures are achieved by the rapid expansion of a high-pressure gas through a thin aperture.15 Because of the low viscosity of argon gas, it can be circulated rapidly within the probe tip. This allows faster cooling at the probe tip, which hastens removal and repositioning (if necessary) of the cryoprobes. After the freezing phase is complete, high-pressure helium gas can then be circulated through the probe tip to cause rapid thawing. Helium provides an effect opposite to that of argon gas under the Joule-Thompson principle, causing a warming of the probe to allow rapid thawing for probe removal from the frozen tissue or repositioning. There are currently two major manufacturers of cryoablation equipment: Galil Medical (Yokneam, Israel) and EndoCare Inc. (Endo Pharmaceuticals, Chadds Ford, Pa.). Both companies use an argon/helium-based system to cool and thaw the probe tip, respectively, and have stand-alone consoles that are connected to gas tanks. Both systems have a similar computer-based interface that demonstrates the vital statistics of the different probes and their respective temperatures (Fig. e143-1 The iceball created during cryoablation is intensely echogenic on ultrasound; this allows real-time monitoring as the iceball forms and propagates. Therefore, damage to the surrounding normal liver parenchyma is limited. The main drawback of ultrasound is the distal acoustic shadow produced by the iceball. Because of the echogenicity of the iceball, ultrasound cannot penetrate beyond it, and structures distal to the iceball are not visualized because of acoustic shadowing. This can be problematic when trying to avoid critical structures that are deep to the tumor being ablated. Reverberation artifact and the critical angle effect are also troublesome when ultrasound guidance is used. The critical angle effect can cause the iceball to appear speciously larger, whereas reverberation causes apparent echoes within the iceball.16 The critical angle effect is due to variation in the speed of sound as it travels through soft tissue versus ice, and is more apparent when using a vector transducer than a linear transducer. CT has many attributes that make it an appealing imaging choice for ablation. CT is widely available and is often primarily used to detect and monitor lesions that have been ablated. CT also shows the anatomy of the liver and adjacent structures well. This allows identification of adjacent structures that may need to be avoided, such as major vessels, the gallbladder, and bowel. In soft tissue, such as liver parenchyma and solid tumors, the iceball is generally well seen as a hypodense region (Fig. 143-3). The margins of cell death and the visible iceball also correlate well when using CT.17–19 One drawback of the use of CT for image-guided ablation is exposure of the patient and personnel to ionizing radiation. In a retrospective study of 20 CT-guided percutaneous liver tumor cryoablations, the total effective dose for the procedure was 72 ± 18 mSv.20 Although that report does suggest ways to reduce such exposures, it calls attention to an added risk with CT guidance. Of course, the uncertain risk due to the radiation is weighed against the imminent health risks associated with the disease state for which the ablation is being utilized.
Cryoablation of Liver Tumors
Indications
Contraindications
Equipment
![]() ). Currently, Galil Medical is the only company to offer a system that is MRI compatible. Galil Medical and EndoCare cryoprobes for percutaneous ablation are needle-like, 17-gauge in size. The Galil system can accommodate up to 25 probes; the EndoCare system up to 8 probes.
). Currently, Galil Medical is the only company to offer a system that is MRI compatible. Galil Medical and EndoCare cryoprobes for percutaneous ablation are needle-like, 17-gauge in size. The Galil system can accommodate up to 25 probes; the EndoCare system up to 8 probes.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cryoablation of Liver Tumors