Temperature
Cellular effect
−5°C to −15°C
Formation of extracellular crystals causing compression and cell deformity
<−15°C slow cool
Efflux of intracellular fluid which results in cellular dehydration and toxic increase in intracellular electrolyte concentration
<−15°C rapid cool
Formation of intracellular crystals causing lysis of intracellular organelles, denaturation of proteins, and cell death
−50 to −80°C
Complete crystallization
Slow thawing
Migratory recrystallization: grinding action of ice movement through the cell
Rapid freezing also causes the formation of intracellular ice crystals that damage intracellular organelles such as the mitochondria and endoplasmic reticulum. This affects the acidity of intracellular fluid leading to further protein and enzyme damage.
Cryotherapy has a significant effect on microcirculation. Cold induces vasoconstriction, endothelial injury, and platelet plug formation leading to microthrombi with subsequent cellular death. This ischemic effect goes beyond the area of direct probe contact to affect the surrounding tissue. It is through this mechanism that malignant tissue, usually hypervascular, is rendered even more sensitive to cryotherapy.
Certain studies suggest that an immune-mediated tumoricidal mechanism through increased activated peripheral lymphocytes even affecting metastatic tumors exists; however, this mechanism is not well elucidated.
The effect of cryotherapy varies, depending on the sensitivity of the treated tissue. This is important when evaluating treatment potential as well as possible complications.
Mazur found the following tissues to be cryoresistant in vitro: fat, cartilage, nerve sheath, connective tissue, and fibrosis. Whereas, tumor, granulation tissue, skin, mucous membranes, nerves, and endothelium are cryosensitive. The inherent sensitivity of the targeted tissue mainly depends on the water content of the tissue. The more water content, the greater the sensitivity. Furthermore, multiple technical aspects influence the outcomes of cryotherapy:
Temperature for tissue destruction. Core tissue temperature must reach between −20°C and −40°C, as rapid freezing of tumors to −40°C or below is associated with 90% cell death.
Rates of freezing and thawing. A process of rapid freezing and slow thawing is the ideal cycle.
Number of freeze-thaw cycles.
The mass of the tissue that is frozen.
The contact area between tissue and cryoprobe.
The effects of cryotherapy on tracheal and bronchial mucosa have been studied in dogs. A return to normal macroscopic mucosa was seen after approximately 2 weeks. Microscopic epithelial and cartilaginous changes took closer to 6 weeks to resolve. The cellular damage and tissue destruction induced by cryotherapy continue over time. It takes hours to days for cellular necrosis to ensue. Necrotic tissue sloughs off within the airways and is often expectorated. Repeated bronchoscopic procedures for removal of debris are generally performed within 5–10 days.
Equipment Used in Endobronchial Cryotherapy
Endobronchial cryotherapy can be performed using a rigid, semirigid, or a flexible cryoprobe. The rigid and semirigid probes can only be used via a rigid bronchoscope, while the flexible probe can be used either through the rigid or flexible bronchoscope (Figs. 33.1 and 33.2).

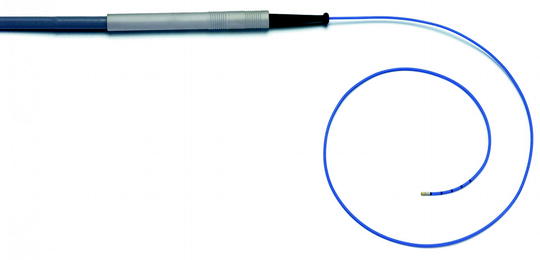

Fig. 33.1
Rigid cryoprobe: Diameter 3 mm and length 530 mm (Source: From erbe-med.com http://www.erbe-med.com/de/medical-technology/public/Products/Cryosurgery/Cryo-Instruments/Probes-and-applicators/Cryoprobes-for-ERBOKRYO-CA/Bronchoscopy-cryoprobe.2448)

Fig. 33.2
Flexible cryoprobe: Diameter 2.4 mm and length 900 mm (http://www.erbe-med.com/de/medical-technology/public/Applications/Pneumology/Tracheabronchial-biopsy-with-flexible-cryo-probe.993)
Cryotherapy units contain a console that regulates the flow of cryogen (Fig. 33.3). This console is typically controlled via foot pedal. Cryoprobes are designed to deliver the highly pressurized liquid cryogen to the tumor. Rigid probes are larger and may contain a reheating system which, in addition to freezing, allows for rapid reheating. This results in a more rapid thawing phase, as compared to that of the flexible probe, where spontaneous thawing must occur, resulting in a longer procedure time.
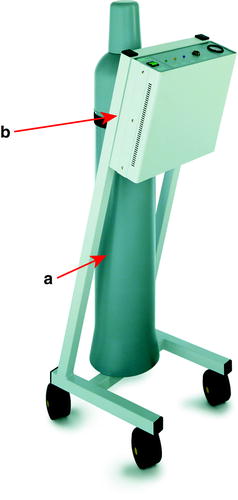

Fig. 33.3
Console and gas cylinder: (a) N2O gas cylinder; (b) cryo-console (http://www.erbe-med.com/de/medical-technology/public/Products/Cryosurgery/Cryosurgery-Units/ERBOKRYO/ERBOKRYO—CA.1154)
Several cooling agents are in use in general cryotherapy. For endobronchial lesions, nitrous oxide (N2O) is the most widely used. Coolants are stored at room temperature under high pressure in liquid phase. The passage of the liquid through the probe from high pressures to atmospheric pressure causes it to expand, resulting in a temperature of −89°C at the tip of the probe.
Indications for Endobronchial Cryotherapy
Cryotherapy is one of multiple methods available to the bronchoscopist. Traditionally, it has been used as a therapeutic tool for the treatment of patients with central airway obstruction. Recently, there is a growing interest in its diagnostic potential, and it is also being used to obtain endobronchial and transbronchial biopsies. When used in the treatment of benign and malignant central airway obstruction, it can be used in lieu of or in conjunction with other modalities of endobronchial treatment, such as mechanical debridement, laser therapy, electrocautery, argon plasma coagulation, brachytherapy, or photodynamic therapy.
Benign Airway Obstruction
Using the cryoprobe for foreign body removal is especially effective when the object has high water content. Mucus plugs, blood clots, aspirated food matter, or pills freeze and adhere well to the cryoprobe, allowing for their extraction frozen to the cryoprobe while the gas is still activated. Objects with a more solid consistency, such as teeth or metal parts, are less cryo-adherent, making removal using the cryoprobe less efficient.
Granulation tissue is highly cryosensitive. Cryotherapy is used especially in the treatment of posttransplantation-, tracheostomy-, or stent-induced granulation tissue formation. It is particularly useful when treating granulation tissue formation in covered metallic stents where other modalities such as electrocautery, argon plasma coagulation, or Nd:YAG laser may be contraindicated.
Removal of benign airway tumors such as endobronchial lipomas and treatment of weblike stenoses has also been reported. However, benign strictures composed mainly of fibrous tissue are not amenable to cryotherapy.
Malignant Endobronchial Disease
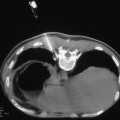
Approximately one third of lung cancer patients have central airway disease on presentation (Fig. 33.4). Their symptoms usually include cough, hemoptysis, dyspnea, and recurrent postobstructive pneumonias.
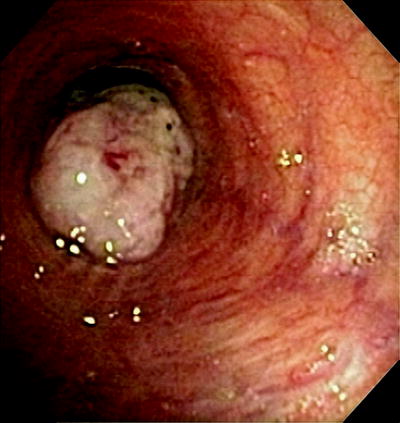

Fig. 33.4
Endobronchial tumor. A 64-year old man with squamous cell carcinoma involving the left mainstem bronchus
Unfortunately, most patients at this stage are inoperable. Systemic chemotherapy is usually ineffective for obstructive disease, particularly in non-small-cell lung cancer. Radiation therapy is slow and is limited by the total dose that can be delivered. It is under these circumstances when endobronchial debridement takes on a major role.
It is important to define the precise role of cryodebridement in lung cancer in order to be able to select the appropriate candidate for cryotherapy. First, a histological diagnosis must be obtained. Surgery with curative intent should be the initial choice. It is only when this goal is not achievable or when palliation is the objective that cryotherapy becomes a viable option.
The patient’s symptoms should be attributable, at least in part, to an endobronchial lesion. The lesion must be accessible to the cryoprobe through the bronchoscope, and the patient should be willing and able to undergo the procedure. Patients with short polypoid lesions with patent distal airways are the ideal candidates for endoscopic intervention.
In patients in respiratory distress, with acute airway emergencies, where immediate recanalization is the main objective, other modalities, such as mechanical debridement, laser therapy, electrocautery, or argon plasma coagulation, are generally preferable, for their immediate effect over cryodebridement, despite recent reports of rapid cryodebridement techniques.
The effectiveness of cryodebridement is limited in longsegment, submucosal, and extrinsic tumors. For mixed tumors, and especially those causing extrinsic compression, treatment with airway dilatation followed by placement of an airway stent is usually warranted. However, cryotherapy is used effectively for the treatment of tumor ingrowth or granulation tissue complicating stent placement.
Cryotherapy Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree