Key Points
- ▪
CT coronary angiography (CTA) has been the principal goal of development of cardiac CT (CCT). The momentum of cardiac CT development has been toward both improving image quality and reducing radiation exposure.
- ▪
CT coronary angiography is able to provide high negative predictive value of significant coronary artery disease.
- ▪
CT coronary angiography is able to quantify coronary artery disease, although with limitations.
- ▪
Current limitations of coronary CT angiography include patient exclusions, coronary calcium, poor ability to characterize small (<2 mm diameter) arteries and branches, and limited outcomes data.
ACC/AHA 17-Segment Coronary Model
Comparative studies of coronary CT angiography (CTA) versus angiographic quantitative coronary analysis (QCA) assessment of coronary anatomy usually are performed comparing the presence or absence of angiographically significant stenosis (>50%) among the “17-segment model” of coronary anatomy.
Imaging Coronary Arteries: Luminal Assessment
Imaging of the coronary artery lumen is a particular challenge for CT scanning, given:
- □
The small luminal size (especially when diseased)
- □
The rapid motion (up to 150 mm/sec, particularly for the arteries in the atrioventricular grooves) through several phases of the cardiac cycle (early systole, early diastole and late diastole—if in sinus rhythm)
- □
The potential of rhythm or rate irregularity during scanning/acquisition
- □
The need for the patient to hold his or her breath during the procedure
- □
The presence of stents and the tendency of diseased coronary arteries to attract calcification—both of which confer problematic signal “blooming” (partial volume averaging) artifacts.
- □
The combination of motion, blooming artifact, and partial signal averaging effect that substantially reduces the depiction of the lumen within calcified and stented coronary arteries
Attaining equivalence in accuracy between CTA and catheter angiography is a challenge that has not been met as of 2014. Catheter angiography has substantially greater imaging resolution (spatial and especially temporal) and hugely greater versatility, yielding clinically adequate images despite calcification and stents, irregular cardiac rhythms, variable cardiac heart rates, and spontaneous or mechanical ventilation, on stable ambulatory patients and patients in cardiogenic shock ( Table 6-1 ).
| CATHETER ANGIOGRAPHY | CT ANGIOGRAPHY | MRI ANGIOGRAPHY | |||
|---|---|---|---|---|---|
| Resolution | |||||
| Spatial (mm) | 0.2 | 0.4–0.75 | 0.7–1.0 mm | ||
| Temporal (ms) | 4–7 | 165–330 Dual source: 60–100 | 0 | ||
| 3D rendering | — | Excellent | Limited | ||
| Experience | Huge | Modest | Limited | ||
| Radiation (mSv) | 3–4 | Newer scanners (mSv) | Older scanners (mSv) | None | |
| Prospective | 0.5–4 | 4–10 | |||
| Retrospective | 4–8 | 12–20 | |||
| Ability to image/characterize the wall | Poor (only dense and thick calcium is apparent) | Good | Poor | ||
Thus, catheter-based angiography has logarithmically greater temporal resolution and several-fold better spatial resolution than contemporary CTA. CTA technology is developing in terms of both spatial and, more importantly, temporal resolution, but the technology gap is notable, and the clinical performance gap is broad in real life. Patients undergoing catheter-based coronary angiography are extremely heterogeneous with respect to many parameters that are relevant to CTA imaging:
- □
Variable heart rates:
- •
Low (due to physiologic, pathologic or pharmacologic reasons) to tachycardic
- •
β-blocker tolerant to β-blocker intolerant
- •
Unstable heart rates
- •
- □
Heart rhythm: normal sinus rhythm to arrhythmia of all forms
- □
Variable potential for artifact:
- •
No calcification or minor calcification to heavy calcification
- •
No stents
- •
Multiple stents, including stenting within stenting
- •
Stenting within calcified areas
- •
Given the inherent lesser spatial resolution of CTA, its best coronary application, and probably its earliest, will be to establish that the lumens of large coronary vessels and bypass grafts are not stenotic.
Magnetic resonance angiography (MRA) is becoming increasingly capable and exhibits comparable sensitivity and specificity and accuracy to coronary CTA (at least the older 16-CT) when compared to QCA for vessels 1.5 mm or larger in very highly selected patients and select centers. Comparison of cardiac magnetic resonance (CMR) with current state-of-the-art equipment does not confirm contemporary equivalence of CMR and CTA. A 3-tesla system with increased signal-to-noise ratio (SNR) allows for increased spatial resolution in whole-heart MRA techniques.
Because the motion, size, and orientation of coronary arteries are different, the accuracy of 64-slice multislice CT (64-MSCT) for the detection of coronary stenoses depends on which vessel is being investigated, due to differences in vessel size and the amount of motion through diastole. The right coronary artery and the left circumflex artery are subject to more motion than the left anterior descending (LAD) artery due to the mechanical effect or motion imparted by atrial contraction ( Table 6-2 ).
| SENSITIVITY (%) | SPECIFICITY (%) | |
|---|---|---|
| Left mainstem | 100 | 100 |
| Right coronary artery | 83 | 100 |
| Left anterior descending coronary artery | 87 | 93 |
| Left circumflex coronary artery | 71 | 77 |
| Total | 84 | 91 |
In patients with normal heart rates, multisegment reconstruction algorithms tend to have superior diagnostic accuracy and image quality compared with half-scan reconstruction algorithms. Automatic selective phase acquisition software in large detector platforms (≥256 slice) and single cardiac cycle acquisition are expected to optimize acquisition with respect to avoidance of diastolic motion and image reconstruction artifacts resulting from multicycle reconstructions.
Calcium, Calcium Scoring, and Calcium Artifact
The presence of calcium, while useful for calcium scoring, is a problem for coronary CTA. The presence of calcium leads to false-positive determination of stenosis as well as overestimation of stenosis. It is more difficult to visualize the lumen in the presence of calcium, due to the “blooming artifact” (i.e., partial volume averaging) of calcium, which is responsible for overestimation of plaque size and stenosis severity. Any motion encountered during acquisition further compounds the blooming and increases the overestimation of stenosis and underestimation of lumen. Blooming artifact is seen less frequently with 64-CCT but remains a significant problem.
The addition of calcium scoring to coronary CTA does not add significantly to the determination of disease. A high calcium score should be seen as a reason not to proceed with CTA, however.
Evidence-Based Review of Coronary Cta
Since about 2005, an accelerating body of evidence has been developing that establishes that coronary CTA is feasible, but within limits that require careful patient selection and preparation. The many limits are represented by the numerous patient exclusions seen in published studies. As the technology has developed, the list of limitation, particularly in terms of patient selection and preparation, has diminished, but exclusions remain numerous and define the nature of use of CTA.
To date, CTA studies have been most useful in excluding disease or classifying disease, if it is present, rather than in performing the vital task of providing a roadmap to plan revascularization procedures in patients with significant disease.
The earliest series using 4-slice CCT equipment, entirely as expected, yielded results inferior to 16-CCT, which currently is considered the bare minimum technology to use for coronary CTA. Today the current standard is 64-CCT or >64-CCT, as presented in the tables in this chapter. Significantly, 64-CCT has not been proven equivalent to conventional angiography. The recent arrival of 256- and 320-CCT, which enable whole heart acquisition in a single cardiac cycle, is expected to provide further imaging benefits, and results of these techniques are eagerly anticipated. Whether they are not just improvements but improvements that render CCT comparable to conventional catheter angiography remains to be seen.
All studies published to date reflect a high degree of selection of patients, establishing that although CTA is feasible, it is not universally feasible, unlike catheter-based techniques. As the temporal resolution of coronary CTA improves, the possibility of including patients with heart rates greater than 75 bpm (who currently are excluded) is emerging, as is the possibility of including patients with irregular heart rhythms. As temporal resolution improves, false-negative CTA studies due to motion artifacts may become less common.
Most comparative studies performed before the introduction of 64-CCT in 2005 are notable for:
- □
Use of a segment-by-segment comparison, which predictably increases the power of the study
- □
Comparison of only vessels ≥1.5 (or ≥2.0) mm in luminal size versus all segments, which is not realistic in excluding CAD or in planning revascularization techniques
- □
Comparison of assessable vessels
More recent studies have strived to include:
- □
All segments, regardless of size
- □
Vessel comparison (single-, double-, triple-vessel disease)
- □
Patient comparison (disease vs. no disease)
To achieve the greatest plausibility and comparative power for conventional coronary angiography, the ideal and most credible studies of coronary CTA will entail no more exclusions than those of coronary angiography and will validate that the use of coronary CTA in the context of potential use of conventional angiography provides a significant outcome or cost benefit.
Summary of CTA for the Detection of CAD
The use of CTA for the detection of CAD is summarized in Figure 6-1 and Tables 6-3 through 6-10 .

| AUTHOR | JOURNAL | YEAR | n | NONASSESS. (%) | SENSITIVITY (%) | SPECIFICITY (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Achenbach et al. | Circ | 2001 | 64 | 32 | 85 | 76 | 59 | 98 |
| Becker et al. | JCAT | 2002 | 28 | 5 | 81 | 90 | 97 | 89 |
| Knez et al. | Am J Card | 2001 | 42 | 6 | 78 | 98 | — | — |
| Kopp et al. | Eur Heart J | 2002 | 102 | 16 | 86 | 96 | — | — |
| Lau et al. | Radiology | 2005 | 50 | — | 79 | 95 | — | — |
| Nieman et al. | Lancet | 2001 | 31 | 27 | 56 | 97 | — | — |
| Nieman et al. | Lancet | 2001 | 31 | 27 | 81 | 97 | — | — |
| Nieman et al. | Circ | 2002 | 59 | 7 | 95 | 86 | — | — |
| Nieman et al. | Heart | 2002 | 53 | 30 | 58 | 76 | — | — |
| Nieman et al. | Radiology | 2003 | 24 | 33 | 85 | 73 | — | — |
| Vogl et al. | Radiology | 2002 | 64 | — | 75 | 91 | — | — |
| Kuettner et al. | JACC | 2004 | 66 | — | 37 | 99 | — | — |
| AUTHOR | JOURNAL | YEAR | n | NONASSESS. (%) | SENSITIVITY (%) | SPECIFICITY (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Ropers et al. | Circ | 2003 | 77 | 12 | 93 | 92 | 79 | 97 |
| AUTHOR | JOURNAL | YEAR | n | NONASSESS. (%) | SENSITIVITY (%) | SPECIFICITY (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Achenbach et al. | Eur Heart J | 2005 | 57 | 5 | 94 | 97 | — | 99 |
| Achenbach et al. | Circ | 2004 | 22 | — | 82 | 88 | — | — |
| Heuschmid et al. | Am J Roent | 2005 | 37 | 0 | 59 | 87 | — | — |
| CAC < 1000 | 93 | 94 | 68 | 99 | ||||
| Hoffmann M et al. | JAMA | 2005 | 103 | 6 | 95 | 98 | — | 99 |
| Hoffmann U et al. | Circ | 2004 | 33 | — | 63 | 96 | 64 | 96 |
| (good image quality) | 82 | 93 | — | — | ||||
| Kefer et al. | JACC | 2005 | 52 | — | 82 | 79 | — | — |
| Kuettner et al. | JACC | 2004 | 58 | 16 | 72 | 97 | — | — |
| (Agatston <1000) | 98 | 98 | — | — | ||||
| Kuettner et al. | Heart | 2005 | 72 | 7 | 85 | 98 | — | 96 |
| Kuettner et al. | JACC | 2005 | 72 | 0 | 82 | 98 | 87 | 97 |
| Martuscelli et al. | Eur Heart J | 2004 | 64 | 16 | 89 | 98 | 90 | 98 |
| Mollet et al. | JACC | 2004 | 128 | 7 | 92 | 95 | 79 | 98 |
| Mollet et al. | JACC | 2005 | 51 | n/a | 95 | 98 | 87 | 99 |
| Morgan-Hughes et al. | Heart | 2005 | 58 | 2 | 83 | 97 | — | 97 |
| Romeo et al. | JACC | 2005 | 53 | 12% transplant pt | 83 | 95 | 71 | 95 |
| Schuijf et al. | Am J Cardiol | 2005 | 45 | 6 | 83 | 97 | — | 97 |
| Cademartiri et al. | Radiol Med (Torino) | 2005 | 60 | 2 | 93 | 97 | 99 | 86 |
| Cademartiri et al. | Am J Roentgenol | 2006 | 38 | 0 | 92 | 96 | 87 | 97 |
| Cademartiri et al. | Radiol Med (Torino) | 2005 | 40 | 0 | 96 | 96 | 86 | 99 |
| Dewey et al. | Invest Radiol | 2005 | 129 | 9 | 83 | 86 | — | 96 |
| Fine et al. | Int J Cardiac Imaging | 2004 | 50 | 2 | 87 | 97 | — | 98 |
| Kaiser et al. | Eur Heart J | 2005 | 149 | 23 | 30 | 91 | — | 83 |
| Aviram et al. | Int J Cardiovasc Intervent | 2005 | 22 | — | 86 | 98 | — | 98 |
| Garcia et al. | JAMA | 2006 | 187 | 29 | 85 | 91 | — | 99 |
| Gulati et al. | Natl Med J India | 2005 | 31 | 14 | 85 | 94 | 76 | 96 |
| AUTHOR | JOURNAL | YEAR | n | NONASSESS. (%) | SENSITIVITY (%) | SPECIFICITY (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Cordeiro et al. | Heart | 2005 | 30 | 20 | 76 | 94 | — | 96 |
| AUTHOR | JOURNAL | YEAR | n | NONASSESS. (%) | SENSITIVITY (%) | SPECIFICITY (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Lim et al. | Clin Radiol | 2005 | 30 | 0 | 99 | 98 | 94 | 99 |
| AUTHOR | JOURNAL | YEAR | n | NONASSESS. (%) | SENSITIVITY (%) | SPECIFICITY (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Leber et al. | JACC | 2005 | 59 | 64 | 97 | |||
| No severe calcium | 87 | 98 | ||||||
| Leschka et al. | Eur Heart J | 2005 | 57 | n/a | 94 | 97 | 99 | |
| Mollet et al. | Circ | 2005 | 52 | 2 | 99 | 95 | 76 | 99 |
| Raff et al. | JACC | 2005 | 70 | 12 | 86 | 95 | 66 | 98 |
| Pugliese et al. | Eur Radiol | 2005 | 35 | 6 | 99 | 96 | 78 | 99 |
| Ropers et al. | Am J Cardiol | 2006 | 84 | 4 | 93 | 97 | 100 | |
| Fine et al. | Am J Cardiol | 2006 | 66 | 6 | 95 | 96 | 97 | 92 |
| Nikolaou et al. | AJR | 2006 | 72 | 6 | 97 | 79 | 96 | |
| Weustink et al. | JACC Per segment | 2007 | 100 1489 100 | 95 [90–97] | 95 [90–97] | 95 [90–97] | 95 [90–97] | |
| Per patient | 95 [90–97] | 95 [90–97] | 95 [90–97] | 95 [90–97] | ||||
| Schuijf et al. | Am J Cardiol | 2006 | 61 | 1 | 85 | 98 | ||
| Ehara et al. | Circ | 2006 | 99 | 8 | 90 | 94 | ||
| Pre-test probability | Post-test negative | Post-test positive | ||||||
| Meijboom et al. | JACC | 2007 | 254 | |||||
| 105 | High probability | 87 | 17 | 96 | ||||
| 83 | Intermediate probability | 53 | 0 | 88 | ||||
| 66 | Low probability | 13 | 0 | 68 | ||||
| Shabestari et al. | Am J Cardiol | 2007 | 35 | 92 | 97 | 77 | 99 | |
| Agatston <100 | 83 | |||||||
| Agatston >400 | 60 | |||||||
| Meijboom et al. | Heart | 2007 | 104 | 92 | 91 | 60 | 99 | |
| Miller et al. 55 | NEJM | 2008 | 291 | Agatston <600, >1.5 mm | 85 [79–90] | 90 [83–94] | 91 [85–95] | 83 [75–89] |
| Budoff et al. | JACC | 2008 | 230 | 1% | ||||
| ≥50% stenosis | 55 | 95 | 83 | 64 | 99 | |||
| ≥70% stenosis | 31 | 94 | 83 | 48 | 99 | |||
| Meijboom et al. | JACC | 2008 | 360 | Per patient | 99 [98–100] | 64 [55–73] | 86 [82–90] | 97 [94–100] |
| Per segment | 88 [85–91] | 90 [89–92] | 47 [44–51] | 99 [98–99] |
| PATIENTS (NO.) | NOT EVALUABLE (%) | SENSITIVITY (%) | SPECIFICITY (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| 701 | 3.8 (27/701) (95% CI: 2.6–5.6) | 98 (398/404) (95% CI: 95–99) | 90 (263/293) (95% CI: 86–93) | 93 (394/4240) (95% CI: 90–95) | 95 (263/273) (95% CI: 93–98) |
| ANALYSIS | NO. | SENSITIVITY (95% CI) | SPECIFICITY (95% CI) |
|---|---|---|---|
| Per segment | 22,798 | 0.81 (0.72-0.89) | 0.93 (0.90-0.97) |
| Per vessel | 2,726 | 0.82 (0.80-0.85) | 0.91 (0.90-0.92) |
| Per patient | 1,570 | 0.96 (0.94-0.98) | 0.74 (0.65-0.84) |
Coronary CTA
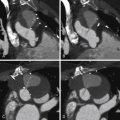
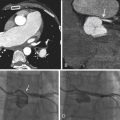
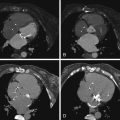
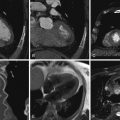
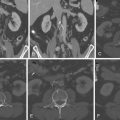
Review of coronary CTA usually begins with a review of axial images and then moves on to maximum intensity projection (MIP) images and reformats (e.g., multiplanar reconstructions and cross-sectional multiplanar reconstructions) to follow the course of the coronary arteries. Workstation software has increasingly included features to assist with coronary (or other) vessel extraction and then depiction. “Seed” markers are placed by the origin of the vessel of interest and distally along its course. Using edge detection algorithms, the vessel is “extracted” and depicted. As the images of the extracted vessel become, to some extent, abstract (for example, a curved artery is depicted in a straightened fashion), reference MIP and 3D images often are displayed concurrently to depict the course of the vessel. These images are usually shown as curved reformations with a single-pixel-thick line coursing down the center of the vessel. Edge detection algorithms allow for maintenance of the center line positioning as the vessel curves. The curve can be represented in any degree of rotation around the center line, which can aid in visualizing eccentric lesions. In addition, cross-sectional “cuts” or planes perpendicular to the long axis of the curved reformations allow for circumferential evaluation of any segment of the analyzed coronary artery ( Figs. 6-2 through 6-23 ; ).








Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree