Case presentation
A 12-hour-old infant presents “looking blue” and with poor feeding per the mother. The child was delivered at home and the mother had no prenatal care. She describes her pregnancy as uneventful. The physical examination reveals an overall well-appearing infant who is afebrile. There is a heart rate of 150 beats per minute, a respiratory rate of 60 breaths per minute, a blood pressure of 68/48 mm Hg, and a pulse oximetry reading of 85% on room air. The lungs are clear. The cardiac examination demonstrates regular rate and rhythm with a harsh III/VI systolic ejection murmur that is difficult to localize in the chest. Capillary refill is approximately 2–3 seconds with 2+ pulses in all extremities.
Intravenous access is difficult to obtain and umbilical artery catheters are placed. An umbilical vein catheter was unable to be placed. Intravenous fluids are ordered, blood is drawn for laboratory tests, antibiotics are ordered, and prostaglandin is ordered to the bedside. Oxygen was administered.
Imaging considerations
Plain radiography
Chest radiography (chest x-ray [CXR]) is utilized as first-line imaging in patients with congenital heart disease (CHD) or in patients ultimately diagnosed with this condition, since the initial symptoms of CHD may mimic respiratory pathology (i.e., tachypnea, hypoxia, crackles, retractions, wheezing). Chest radiography has been found to have a low sensitivity for structural heart disease (26%–59%), a negative predictive value of 46%–52%, and a lower sensitivity in premature infants, less than 35 weeks’ gestation. The use of chest radiography as a routine screening test for CHD is not supported, and patients with unremarkable radiography in the clinical context of suspected CHD should have echocardiography. The performance of CXR in the detection of cardiac enlargement in pediatric patients has been compared to a gold standard of cardiac enlargement by echocardiography. CXR was found to have high specificity and negative predictive value (92.3% and 91.1%, respectively) for cardiac enlargement, but low sensitivity and positive predictive value (58.8% and 62.5%, respectively). The identification of cardiomegaly by plain chest radiography in adults has also been shown to have low sensitivity (40%) but high specificity (91%). The cardiothoracic ratio has been used as a marker for cardiac size in adult and pediatric patients; in adult patients, a cardiothoracic ratio greater than 0.55 has been shown to correlate with an increased risk of death.
Plain chest radiography, however, does have value. Causes of respiratory distress, such as pneumonia or pneumothorax, can be detected by this modality. Additionally, ionizing radiation exposure is minimal, sedation is not required, and radiographic imaging is rapid and readily available.
Computed tomography (CT)
This noninvasive modality is useful to image vascular and extracardiac structures, especially those with limited visualization on echocardiography. , CT angiography has been utilized to visualize cardiac anatomy, the coronary arteries, and other vascular structures. CT angiography is very useful in evaluating major arteries and veins, the aortic arch, and vascular rings. The relationship of vascular and airway structures can also be evaluated. , Compared to cardiac magnetic resonance imaging (MRI), CT angiography has superior spatial resolution (allowing for more detailed visualization of small vessels), is preferred when there are airway or pulmonary abnormalities, and may be used in patients in whom MRI may be contraindicated, such as patients with a pacemaker, internal defibrillator, or aneurysm clip.
CT does involve exposure to ionizing radiation and use of intravenous contrast material. While sedation is generally not necessary, it may at times be needed. CT is a readily available modality and can rapidly produce useful images for diagnosis and management, but CT for CHD should be performed and interpreted by those with expertise in pediatric CT imaging for CHD to obtain a diagnostic study and a meaningful interpretation.
Magnetic resonance imaging
MRI and magnetic resonance angiography (MRA) have proven to be excellent imaging modalities for patients with CHD, in both preoperative and postoperative patients. MRI can be utilized to assess anatomy and physiologic function and has the ability to assess for multiple conditions, including tetralogy of Fallot (TOF), transposition of the great vessels, single ventricle physiology, cardiac tumors, myocarditis, and cardiomyopathies. , , Not all cardiac MRI studies require intravenous contrast. Gadolinium-based contrast agents are the typical contrast agents utilized. Contrast enhancement facilitates visualization of vascular structures and allows performance of cardiac function and perfusion studies. In patients with cardiomyopathies, right ventricular dysplasia, and myocarditis, contrast-enhanced MRI studies can be used to detect scarring or fibrosis. ,
One disadvantage of cardiac MRI is the length of time needed to complete a study, and breath holds or sedation may be required. Some young infants may not require sedation, as they may be swaddled. , ,
Ultrasound (US)
Echocardiography continues to be a first-line imaging modality in pediatric patients with suspected CHD. Excellent visualization of cardiac structure, anatomy, and function can be achieved with echocardiography. , Echocardiography can be utilized to diagnose structural cardiac disease and plan for operative repair and to clinically follow patients.
Imaging findings
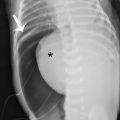
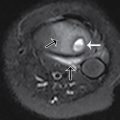
A single frontal view plain radiograph of the chest is obtained. There is cardiomegaly with normal pulmonary vascularity by chest radiograph. The lungs do not show focal consolidation or pneumothorax ( Fig. 8.1 ).

Case conclusion
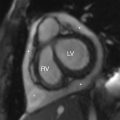
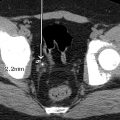
The child was somewhat responsive to oxygen administration and the oxygen saturations improved. The laboratory tests were not suggestive of infection, but antibiotics were administered due to the lack of prenatal care and the possibility of sepsis. While consideration was given to a metabolic syndrome as the cause of the patient’s symptoms, a cardiac etiology was also considered. An emergent echocardiogram was performed, showing anatomy consistent with TOF with an absent pulmonary valve. The patient was admitted to the pediatric cardiac intensive care unit and consultation with pediatric cardiology and pediatric cardiothoracic surgery was obtained. Appropriate management was provided and the patient was able to be discharged from the hospital with close monitoring and follow-up. At 4 months of age, the patient had CT angiography performed, and selected images are provided here. This study demonstrates findings consistent with TOF with absent pulmonary valve, including marked dilatation of the right and left main pulmonary arteries ( Fig. 8.2 ). Although pulmonary artery dilatation is not a common feature of TOF, it is a characteristic finding of TOF with absent pulmonary valve, due to chronic pulmonary regurgitation. CT also demonstrates an aortic coarctation just beyond the origin of the left subclavian artery ( Fig. 8.3 ).



At 6 months of age, the patient underwent repair of the cardiac defects. He was hospitalized for several weeks but did well.
The incidence of CHD is approximately 4–10 in 1000 live births. , , The diagnosis of CHD in pediatric patients can be challenging. The presenting symptoms may mimic a variety of conditions, including viral upper respiratory infection, metabolic illness, dehydration, pneumonia, and sepsis. A history of cyanosis, increased work of breathing, and sweating or rapid tiring with feeding are concerning for a cardiac lesion. Tachypnea and hepatomegaly are signs of CHD but are not immediately present, nor present in all cases of CHD. Attention should be given to palpation of the femoral pulses. Weak or absent femoral pulses suggest coarctation of the aorta or an interrupted aortic arch.
The hyperoxia test has been used as a quick method to discern whether hypoxia in a neonate is cardiac or pulmonary in origin. The test is performed by providing 100% oxygen for at least 10 minutes. No improvement with this maneuver suggests cardiac disease; in an abnormal (positive) test, the partial pressure of oxygen (PaO 2 ) will not rise above 100 mm Hg (and usually remains less than 60 mm Hg). ,
Another useful physical examination tool that may be implemented to ascertain if there is coarctation of the aorta or an interrupted aortic arch is the measurement of the blood pressure in the four extremities. A difference of more than 20 mm Hg between upper and lower extremity blood pressures suggests an aortic obstructive lesion between the arterial supply of the upper and lower extremities, including aortic coarctation or an interrupted aortic arch. ,
Prostaglandin is an essential medication used to maintain the patency of the ductus arteriosus. Since infants with ductal-dependent heart lesions can decompensate quickly, it is essential to maintain ductal patency to stabilize the child until a definitive diagnosis can be made. Prostaglandin E1 (alprostadil) is used for this purpose in pediatric patients. These substances dilate the smooth muscle of the ductus arteriosus and can be delivered through intravenous or intraosseous routes. In infants with suspected ductal-dependent CHD, initiation of prostaglandin should not be delayed. The dose is titrated to effect; one should see improvement in oxygenation (75%–85%) and pulses. Potential side effects of prostaglandin administration include apnea and fever (very commonly seen); hypotension, tachycardia, lower seizure threshold, hypothermia, and cardiac arrest have been reported but are very rare. The clinician should anticipate these potential adverse effects and address them appropriately.
In 2011, the Routine Universal Screening Program was implemented in the majority of states in the United States and the District of Columbia to screen newborns for critical CHD prior to discharge from the hospital. , The purpose of this program is to screen newborns for critical CHD typically discovered in the newborn period, such as critical coarctation of the aorta, TOF, truncus arteriosus, tricuspid atresia, total anomalous pulmonary venous return (TAPVR), pulmonary atresia, and transposition of the great vessels, among others, although a failed screen may also indicate a pulmonary process, such as pulmonary hypertension. This screen is easily accomplished with pulse oximetry, noting differences between the extremities, and is performed after 24–48 hours of age. Infants with a failed screen should have chest radiography, an electrocardiogram (ECG), and echocardiography. , A significant percentage of infants, though, will be discharged from the hospital with undiagnosed CHD.
| LESION | CLINICAL SIGNS | INITIAL DIAGNOSIS | MANAGEMENT |
| Tetralogy of Fallot VSD Overriding aorta RV outflow obstruction RV hypertrophy | Harsh pulmonary ejection murmur “Tet” spells | Echocardiogram “Tet” spells CXR—“boot”-shaped heart—not sensitive or specific; concave main pulmonary artery segment Decreased pulmonary circulation | “Tet” Spell: Calming maneuvers/O 2 IV fluids Morphine Surgical repair |
| Transposition of the great vessels | Cyanosis (usually early) Usually no murmur | Echocardiogram CXR—narrow superior mediastinal silhouette “egg on a string” | Surgical repair |
| Truncus arteriosus | Pulmonary overcirculation Loud systolic murmur | Echocardiogram CXR—pulmonary overcirculation | Diuresis Fluid restriction Surgical repair |
| TAPVR | Cyanosis (usually early) Respiratory distress Pulmonary overload | Echocardiogram CXR—overcirculation or pulmonary venous hypertension, if severe heart failure, “whiteout” of lung fields, cardiac silhouette variable size according to type; snowman mediastinum (supracardiac TAPVR) | Surgical emergency |
| Hypoplastic left heart syndrome | Cyanosis as the PDA closes Poor urine output Poor pulses Mottling Respiratory distress | Echocardiography CXR—pulmonary vascular congestion Congestive heart failure Possible cardiomegaly | Initial stabilization (prostaglandin E1) Staged palliative repair |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree