Anatomy, embryology, pathophysiology
The term adnexa includes the fallopian tubes, the ovaries, and their ligamentous attachments in the female pelvis.
The fallopian tubes
- ◼
The fallopian tubes are paired tubular structures, approximately 10 cm in length extending from the uterus to the ovaries.
- ◼
The fallopian tubes consist of four parts. From medial to lateral, these are the isthmus, ampulla, infundibulum, and fimbriae. The fimbrial segments overhang the ovary and communicate with the peritoneal cavity.
- ◼
The fallopian tubes are contained within two folds of the broad ligament.
The ovaries
- ◼
The ovaries lie within the lateral pelvic sidewalls in the ovarian fossae. Normal ovaries are paired ovoid structures, roughly measuring 4 × 3 × 2 cm, although their volume varies with age, menopausal status, and menstrual cycle.
- ◼
The ovary is surrounded by an outer fibrous layer of tunica albuginea. Its internal structure is comprised of three zones—an outer cortex, inner medulla and a hilum. The cortex contains follicles, corpus lutea, fibroblasts, and smooth muscle cells.
- ◼
The ovary has two functions: hormone production and oocyte production (folliculogenesis). Oocyte precursors present from birth are known as primordial germ cells. Following puberty, these mature under the influence of follicle stimulating hormone and luteinizing hormone. In each cycle, approximately 20 follicles are activated, with usually just one follicle fully maturing, while the others contribute to endocrine function.
- ◼
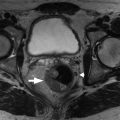
On magnetic resonance imaging (MRI), ovaries are recognized as ovoid structures containing T2 hyperintense physiologic follicles with intervening T1 hypointense stroma ( Fig. 33.1 ).
Fig. 33.1
Normal ovaries. Normal ovaries averaging 10 mL in size with subcentimeter ovoid, peripheral, subcentimeter follicle cysts ( thin arrows ) and central stroma ( thick arrows ) are easily identified. This is exemplified in the axial (A) and coronal (B) T2-weighted images. A small, partially subserosal fibroid protrudes from the anterior uterine body ( open arrow in B).
(From Roth C, Deshmukh S. Fundamentals of Body MRI , ed 2. Philadelphia: Elsevier; 2016.)
- ◼
On computed tomography (CT), they have soft tissue attenuation and may contain a follicle or corpus luteal cyst.
Ligamentous pelvic structures
- ◼
The broad ligament is a sheet of peritoneum that extends from the uterine body to the lateral pelvic walls and has three components: mesometrium, mesovarium, and mesosalpinx.
- ◼
The caudal extent of the broad ligament is defined by the cardinal ligaments.
- ◼
The broad ligament is usually not visible on imaging unless there is pelvic ascites.
- ◼
The broad ligament contains the ovarian ligament, round ligament of the uterus and suspensory ligament of the ovary.
Blood supply and lymphatic drainage of the adnexa
- ◼
The ovaries have a dual arterial blood supply. The main arterial supply comes from the ovarian artery, a branch of the abdominal aorta arising at the L2 vertebral level. Collateral supply comes from the ovarian branches of the uterine artery.
- ◼
The ovarian vein is typically single, but may also be multiple and accompanies the ovarian artery. The left ovarian vein drains into the left renal vein, and the right ovarian vein drains into the inferior vena cava. The ovarian lymphatics drain into the paraaortic lymph nodes at the level of the lower pole of the kidneys.
Techniques
Hysterosalpingogram
A fluoroscopic technique for assessing the uterine cavity and fallopian tubes whereby a catheter is inserted into the cervix under direct visualization. Contrast is then slowly instilled via the catheter under serial fluoroscopy. The primary aim of the study is to confirm tubal patency and to identify uterine anomalies.
Transabdominal and transvaginal ultrasonography
Pelvic ultrasound is the first-line imaging modality for assessment of the adnexa. It offers many advantages including the noninvasive nature of the test, the absence of ionizing radiation exposure, low cost, and wide availability. Transabdominal pelvic sonography uses the distended urinary bladder as an acoustic window for visualization of the pelvic and adnexal structures. The probe is typically of low to medium frequency (e.g., 5 MHz). Transvaginal/endovaginal US provides higher resolution (approximately 7 MHz) and is thus preferable where appropriate.
Multiparametric magnetic resonance imaging
A sonographically indeterminate mass may be further evaluated with MRI. MRI provides additional information on the composition of soft-tissue masses using differences in MR relaxation properties seen in various types of tissue. MRI can differentiate fat, blood, fibrous tissue, and vascularized tumor. The large field of view and multiplanar sequences can also help determine the origin of the mass.
Computed tomography
CT is rarely a primary assessment tool for adnexal imaging. Disadvantages include ionizing radiation exposure and poorer soft tissue differentiation as compared with MRI. The primary role of CT in adnexal imaging is in staging malignancy.
Protocols
Protocol considerations
Pelvic MRI is typically performed with the patient positioned supine using a phased array coil. Axial imaging planes are helpful for assessment of pelvic and parametrial anatomy. The sagittal plane provides information on zonal anatomy of the uterus. Oblique imaging is helpful in assessment of uterine anomalies. Typically, an initial localizer sequence is obtained in the coronal place using a fast sequence such as a single shot fast spin-echo sequence. High-resolution T2 sequences should be acquired in three planes (sagittal, axial, coronal) without fat suppression to delineate anatomy. A T1-weighted nonfat-suppressed sequence or an opposed phase T1-weighted sequence is useful for the identification of fat in a lesion (e.g., teratoma). A T1-weighted sequence with fat suppression is used for the detection of blood products, for example, endometrioma and for correlation with postcontrast sequences to look for intralesional enhancement. Axial T1-weighted sequences are extended into the upper abdomen to assess for nodal pathology.
Suggested protocol
- ◼
Large field of view (FOV) coronal T2 single shot fast spin echo without fat suppression from kidneys to pelvis.
- ◼
Axial T1 turbo spin echo (TSE) without fat suppression from iliac crest to perineum.
- ◼
Axial T2 TSE without fat suppression from iliac crest to perineum.
- ◼
Sagittal T2 TSE without fat suppression from mid-femoral head to mid-femoral head.
- ◼
Coronal T2 TSE without fat suppression from sacrum to anterior abdominal wall.
- ◼
Axial T2 TSE oblique to the long axis of the uterus to assess for a Müllerian anomaly.
- ◼
Axial T1 precontrast with fat suppression from the iliac crest to the perineum.
- ◼
Axial T1 postcontrast with fat suppression from the iliac crest to perineum.
- ◼
Sagittal and coronal postcontrast with fat suppression to match T2 FOV.
- ◼
Axial diffusion-weighted imaging (B0, B500, and B1000) from iliac crest to perineum.
Specific disease processes
Nonneoplastic cystic adnexal lesions
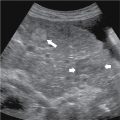
Functional/follicular cyst: this refers to the persistence of an unruptured Graafian follicle. These are anechoic, thin–walled, unilocular structures larger than 3 cm (a follicle is <3 cm) without any internal echoes, solid component, or internal vascularity ( Figs. 33.2 and 33.3 ).
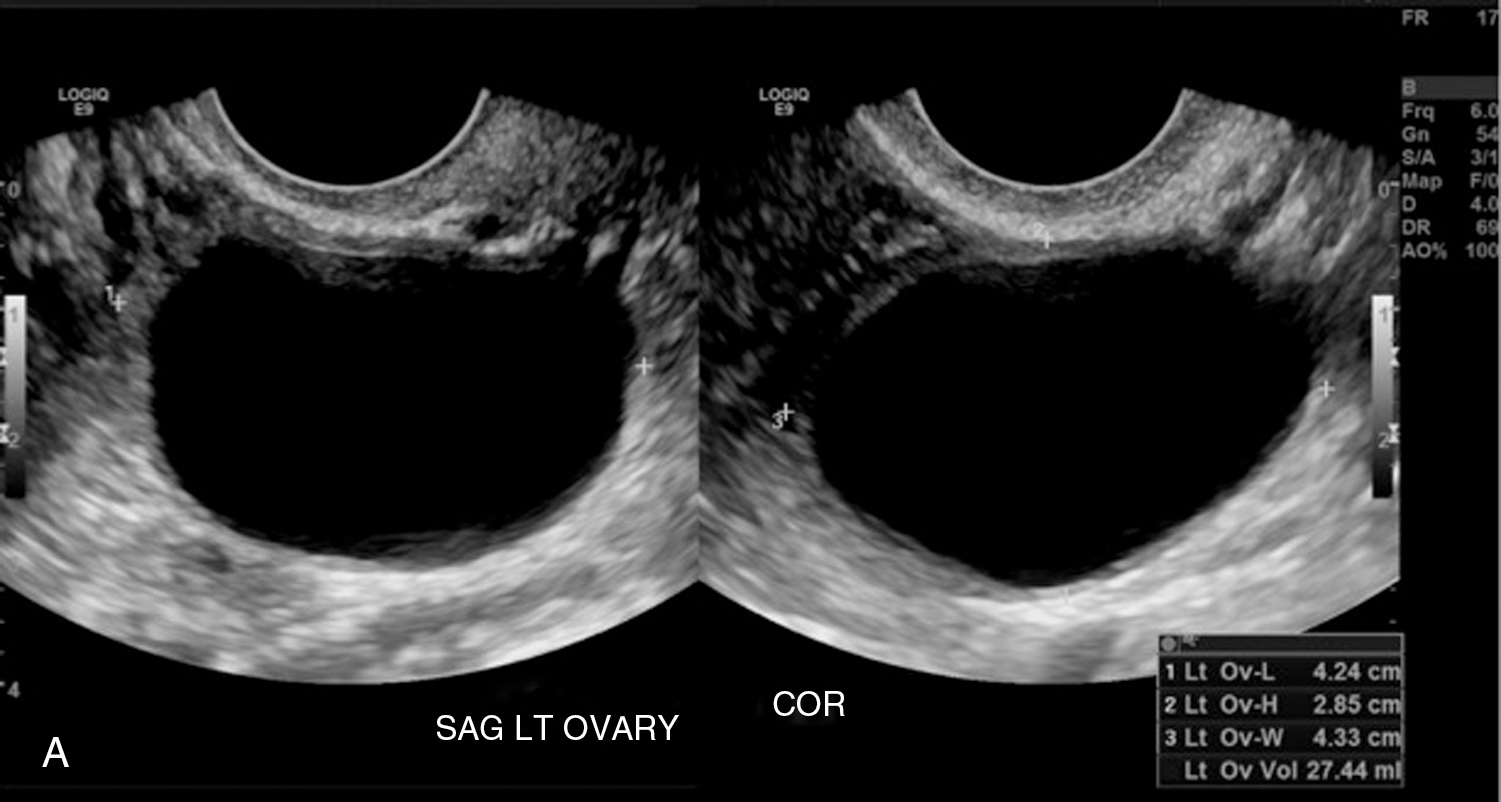
Fig. 33.2
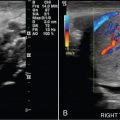
Simple ovarian cyst: a 41-year-old female with left lower quadrant pain. Gray-scale (A) and color flow (B) ultrasound images demonstrate an anechoic left ovarian lesion without any internal complexity, compatible with a simple cyst.
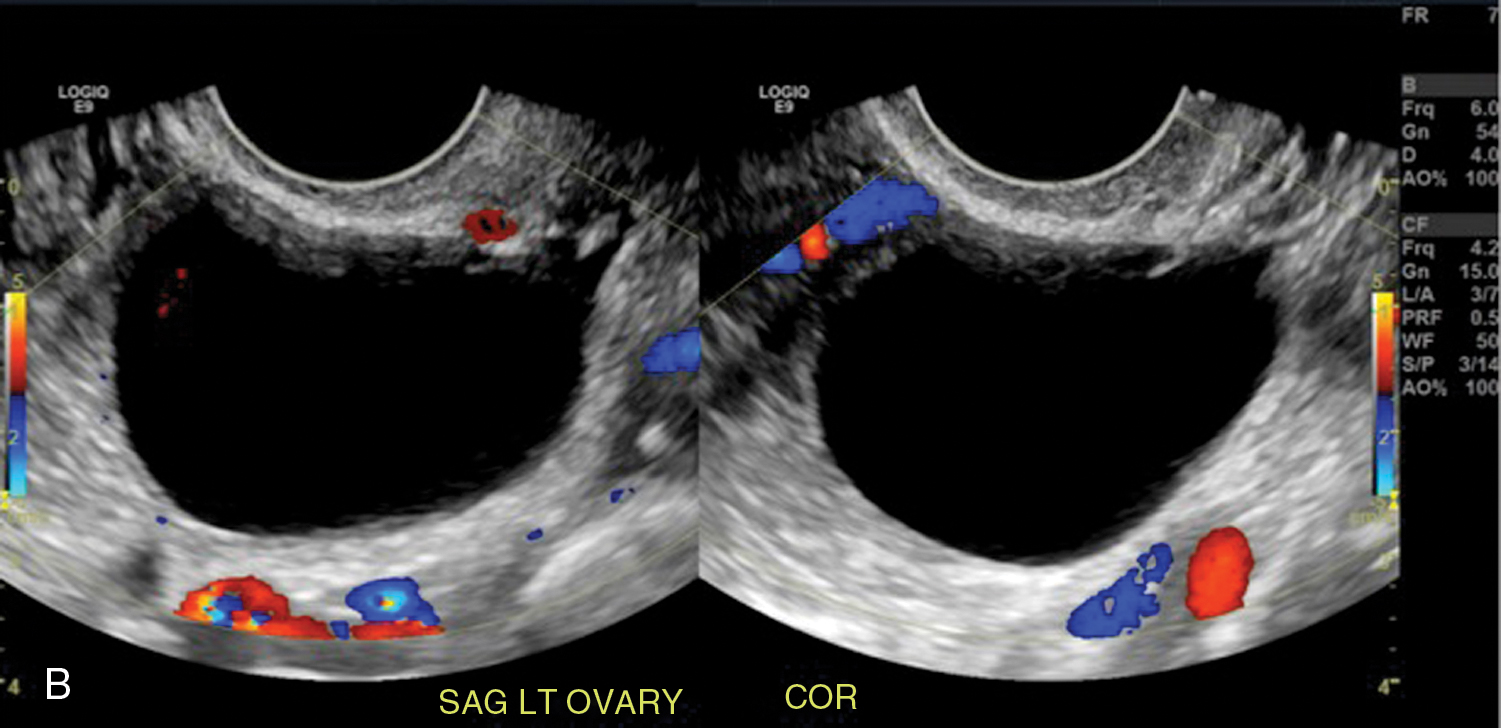

Fig. 33.3
Simple ovarian cyst. Axial T2-weighted (A), T1-weighted (B), precontrast (C), and postcontrast (D) T1-weighted fat-suppressed images show a small, simple right-sided ovarian cyst ( thin arrow in A–D) exhibiting simple fluid characteristics with no complexity or enhancement and coexisting with a probable left ovarian corpus luteal cyst ( thick arrow in A–C) with a thin rim of T1 hyperintensity (blood) and otherwise simple cystic features. With increased size (especially >5 cm) and complexity, the probability of neoplasm escalates as illustrated in the axial T2-weighted image of a different patient (E) who has a benign ovarian cystic epithelial neoplasm with septation ( thin arrows ) and mild eccentric wall thickening ( thick arrow ).
(From Roth C, Deshmukh S. Fundamentals of Body MRI , ed 2. Philadelphia: Elsevier; 2016.)
Corpus luteum cyst: the corpus luteum fails to regress following release of the ovum ( Fig. 33.4 ). Variable appearance on US depending on presence or absence of intracystic hemorrhage. Typical features include:

Fig. 33.4
Corpus luteal cyst. Coronal T2-weighted (A) and axial fat-suppressed enhanced T1-weighted (B) images show a small cystic lesion ( thin arrow in A) with a thin peripheral rim of enhancement ( thick arrows in B) and no other evidence of complexity. C–F, In a different patient, a thicker rim of enhancement delineates a right sided corpus luteal cyst ( arrow ) in the T2-weighted (C), T1-weighted (D), enhanced T1-weighted unsuppressed (E), and fat-suppressed (F) images. G, An irregular—collapsed or deflated—morphology often characterizes corpus luteal cysts, as seen in a different patient ( arrow ).
(From Roth C, Deshmukh S. Fundamentals of Body MRI , ed 2. Philadelphia: Elsevier; 2016.)

- ◼
Size under 3 cm.
- ◼
Cystic lesion with crenulated walls and internal echoes.
- ◼
Iso-hypoechoic solid appearing ovarian lesion because of hemorrhage.
- ◼
Centrally avascular with peripheral “ring of fire” on color Doppler US.
Hemorrhagic cyst: likely because of bleeding into a corpus luteum. The majority of these lesions have readily recognizable ultrasound features. Typical appearance is a reticular pattern of internal echoes because of fibrin strands. This pattern is frequently referred to as “fishnet”, “lacy”, “cobweb” or a “spiderweb” pattern ( Fig. 33.5 ). Clot in a hemorrhagic cyst can sometimes mimic a solid nodule.
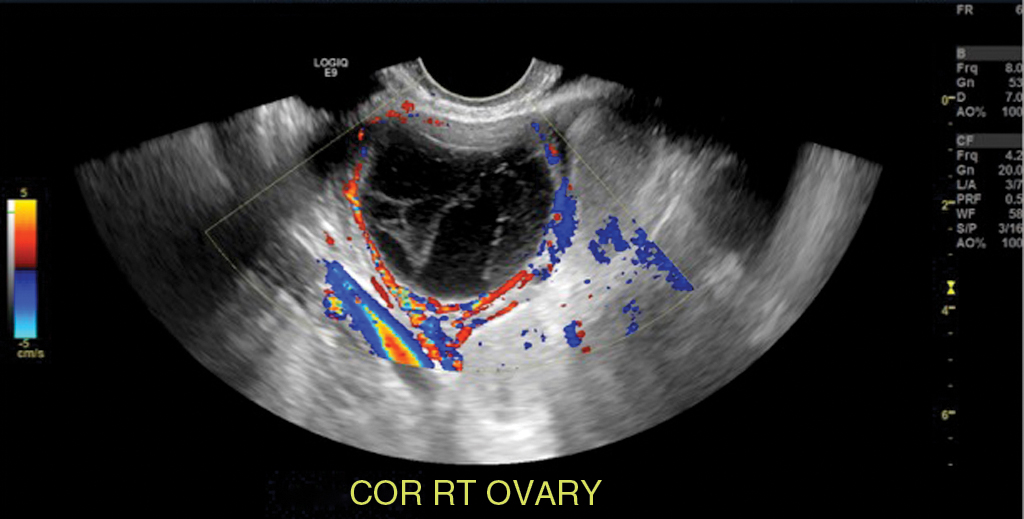
Fig. 33.5
Hemorrhagic ovarian cyst: A 30-year-old, otherwise well female presenting with a 5-day history of suprapubic and right lower quadrant pain. Gray-scale and color flow images of a typical ovarian hemorrhagic cyst, with a reticular pattern of thin internal echoes because of fibrin strands. Follow-up ultrasound at 6 weeks demonstrated complete interval resolution.
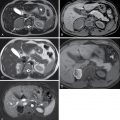
Endometrioma: ectopic endometrial tissue outside the uterine cavity. Appearance on sonography can be variable. Typically, it is a unilocular cyst with diffuse homogeneous ground-glass echoes and posterior acoustic enhancement ( Fig. 33.6 ). The internal echoes are because of hemorrhagic debris within the cyst. When atypical features are present (e.g., multiple locules or a solid component) MRI should be performed. Typically, they demonstrate intrinsic T1 signal hyperintensity on MRI ( Fig. 33.7 ). They do not lose signal on opposed phase imaging (unlike teratoma) ( Fig. 33.8 ).

Fig. 33.6
Endometrioma on computed tomography (CT) and ultrasound. A, A cystic lesion is identified in the left adnexa on CT in a young woman with pelvic pain. This lesion was present on a CT 1 year before (not shown) and thought at that time to represent a functional cyst. B, Ultrasound obtained to further characterize this persistent lesion shows a cystic mass in the left ovary with posterior acoustic enhancement and diffuse low-level internal echoes, findings characteristic of an endometrioma.
(From Zagoria RJ, Brady CM, Dyer RB. Genitourinary Imaging: the Requisites , ed 3. Philadelphia: Elsevier; 2016.)
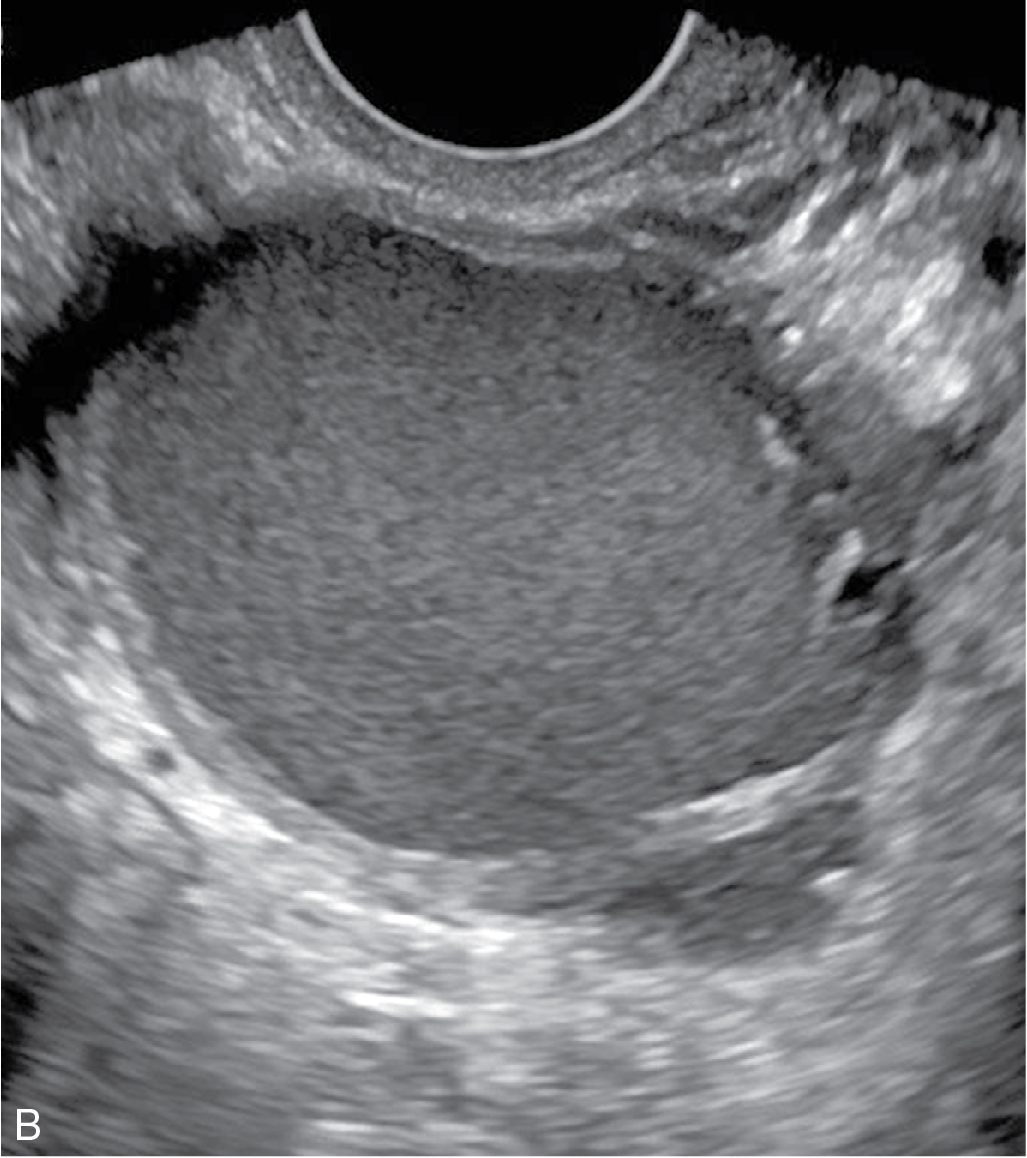

Fig. 33.7
Endometriomas. Another typical case of endometriosis reveals an ill-defined left adnexal lesion with marked T2-shortening ( arrow in A) combined with corresponding hyperintensity in the T1-weighted fat-suppressed image (B)—signifying concentrated hemorrhage—lesion multiplicity, and nonspherical morphology. C–E, In a different patient, multiple irregular lesions ( arrows in C and E) with similar signal characteristics in the T1-weighted fat-suppressed image (C) with shading—albeit less profound—in the T2-weighted image ( arrows in D) typify endometriosis. (E) The T1-weighted in-phase gradient echo image reveals additional bilateral hyperintense lesions in the iliac fossa ( thick arrows ), not to be confused with endometriomas (or other hemorrhagic or fatty lesions). Flow-related enhancement accounts for hyperintensity in the iliac veins, in this case. Remember that gradient echo images are time-of-flight images (without the parameter modifications of dedicated vascular sequences) and prone to the in-flow effect (especially in two-dimensional sequences in the case of the entry slice with respect to the vessel).
(From Roth C, Deshmukh S. Fundamentals of Body MRI , ed 2. Philadelphia: Elsevier; 2016.)

Fig. 33.8
Magnetic resonance imaging of ovarian dermoid and endometrioma. A, Two masses in the pelvis are hyperintense on a T1-weighted gradient-echo in-phase image. The anterior mass is isointense to subcutaneous fat and the posterior mass is hyperintense relative to the subcutaneous fat. B, On a T1-weighted image with fat saturation, the anterior mass loses signal secondary to the presence of macroscopic fat, whereas the posterior lesion remains hyperintense secondary to blood products. Findings are consistent with an ovarian dermoid anteriorly and an endometrioma posteriorly. C, The dermoid is isointense to fat on a T2-weighted image without fat suppression and the endometrioma shows marked signal loss (T2 shading).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree