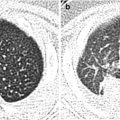
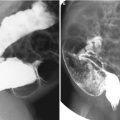
Fig. 24.1
Autosomal recessive polycystic kidney disease in a 7-day-old boy. (a) Longitudinal US shows an enlarged kidney with heterogeneously increased echogenicity. Corticomedullary differentiation is obliterated. (b) High-resolution US of the kidney shows radially oriented linear echogenicity within the renal medulla, indicating ectatic tubules. Note relative sparing of the renal cortex. (c) Follow-up US after 5 years shows multiple tiny echogenic foci and small cysts with obliteration of the corticomedullary differentiation
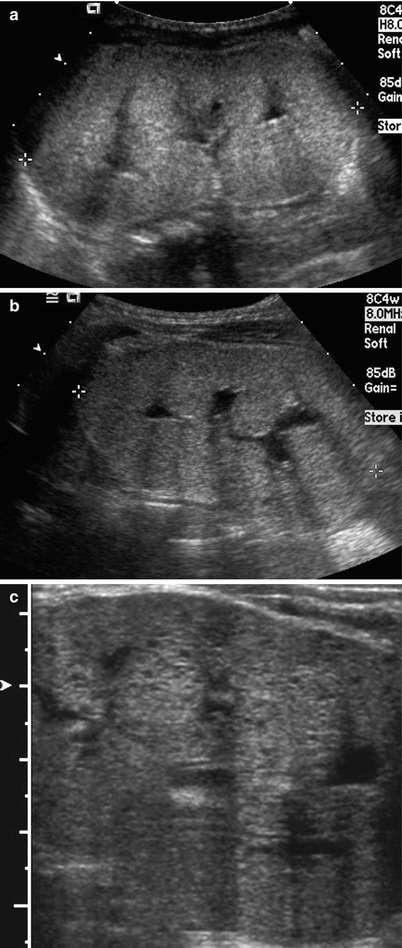
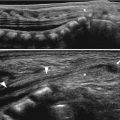
Fig. 24.2
Autosomal recessive polycystic kidney disease in a 1-day-old girl. (a and b) Longitudinal US images of right kidney (a) and left kidney (b) show diffuse echogenic foci with obliteration of the corticomedullary differentiation. (c) High-resolution US of the kidney demonstrates multiple tiny cysts within echogenic renal parenchyma
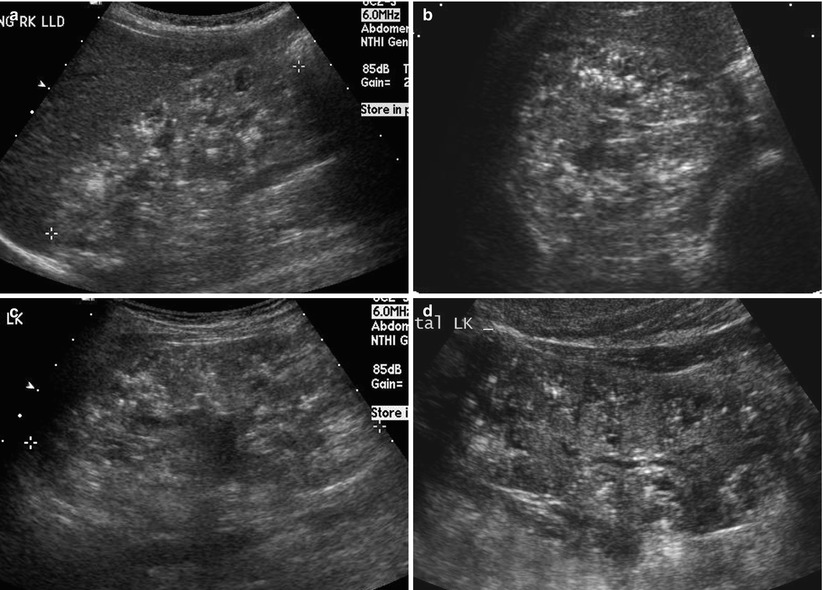
Fig. 24.3
Autosomal recessive polycystic kidney disease in a 7-year-old boy. (a and b) Longitudinal (a) and transverse (b) US images of right kidney show multiple tiny echogenic foci and small cysts with obliteration of the corticomedullary differentiation. (c) Longitudinal US of the left kidney shows numerous tiny echogenic foci and small cysts. Note that the peripheral renal cortex is relatively spared. The renal pelvis is seen. (d) Two years later, multiple tiny echogenic foci are identified mainly within the medullary regions. The peripheral renal cortex is more spared as compared to prior US images
24.4.2 Autosomal Dominant Polycystic Kidney Disease
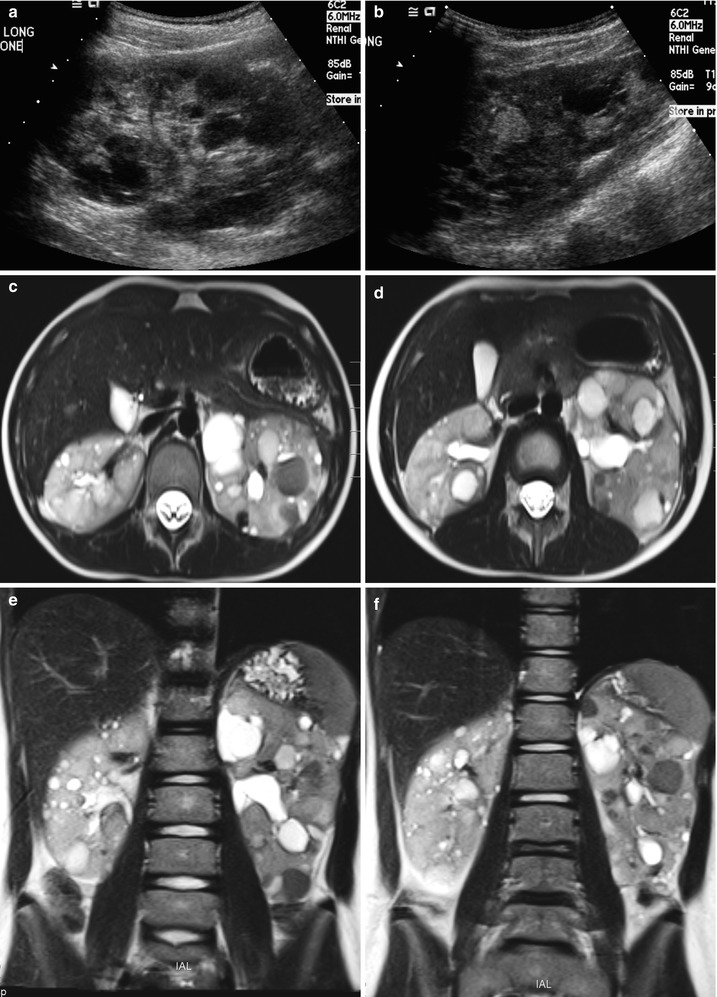
Fig. 24.4
Autosomal dominant polycystic kidney disease in a 6-year-old girl. (a and b) Longitudinal US of the left kidney demonstrates multiple, variable-sized hypoechoic cysts. Note multiple echogenic round cysts, which represent hemorrhagic cysts. (c and d) T2-weighted axial MR images demonstrate variable-sized cysts with variable contents within the cysts. Note multiple low signal intensity cysts with fluid-fluid level, which represent hemorrhagic cysts. (e and f) T2-weighted coronal MR images show marked enlargement of both kidneys with variable-sized cysts occupying the entire kidney
24.4.3 Multicystic Dysplastic Kidney
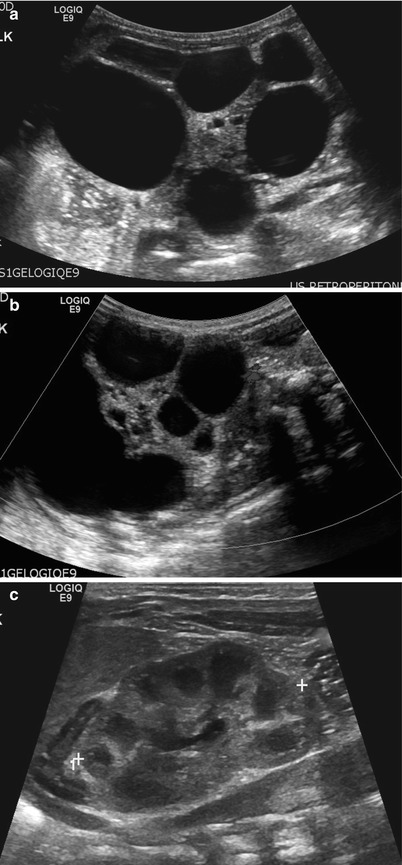
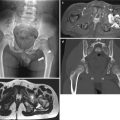
Fig. 24.5
Multicystic dysplastic kidney in a 1-day-old girl. (a) Longitudinal US of the left kidney shows multiple cysts of varying size and intervening hyperechoic area. Left kidney measures about 9.4 cm in length. Normal renal parenchyma is not demonstrated. (b) Color Doppler US of the left kidney shows no vascular structure within the intervening hyperechoic area. (c) Longitudinal US of the right kidney shows normal renal parenchyma. Right kidney measures about 4.0 cm in length
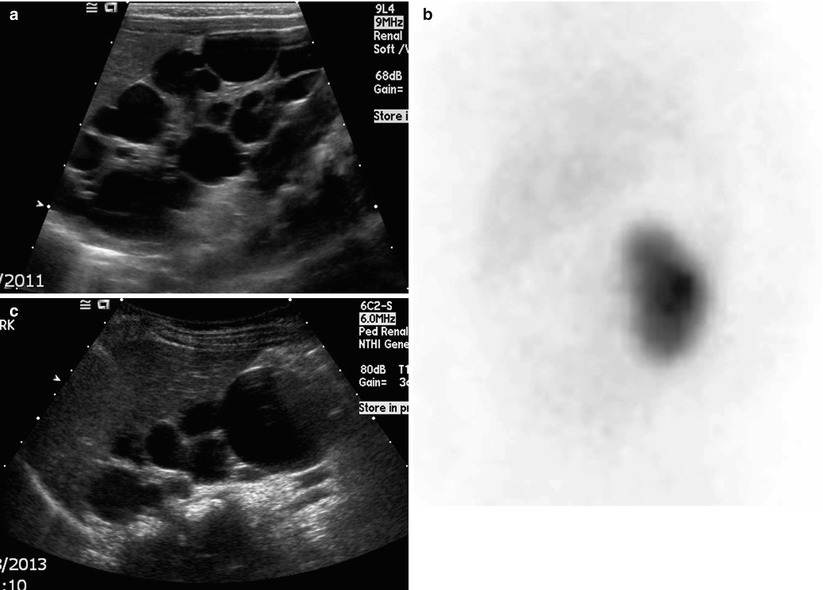
Fig. 24.6
Multicystic dysplastic kidney in a 1-month-old girl. (a) Longitudinal US of the right kidney shows multiple cysts without normal renal parenchyma. (b) An anterior view of DMSA renal scintigraphy shows no uptake of radioisotopes in the right kidney. (c) Two years later, most of the cysts have decreased in size and number with the exception of a large cyst in the lower pole
24.4.4 Multiple Malformation Syndromes with Renal Cysts
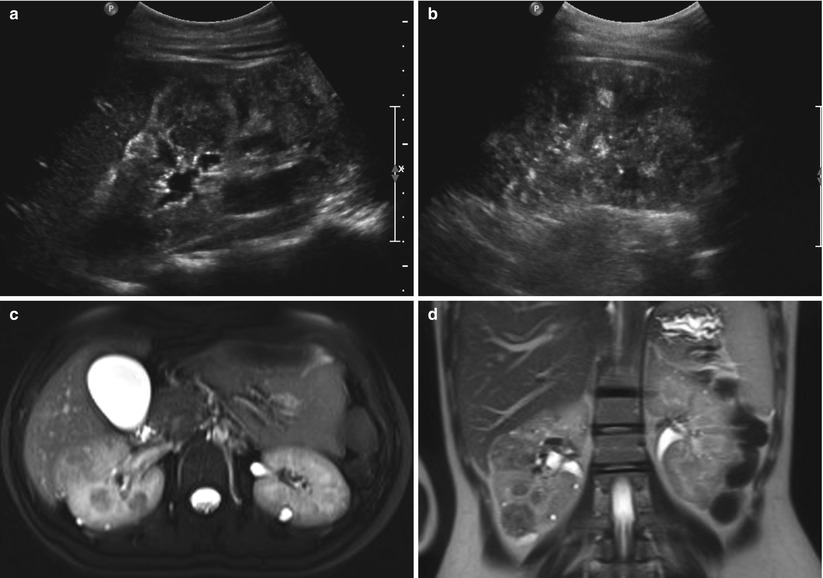
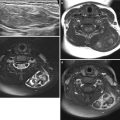
Fig. 24.7
Angiomyolipomas and renal cysts in a 17-year-old girl with tuberous sclerosis. (a and b) Longitudinal US of both kidneys shows multiple echogenic round angiomyolipomas and hypoechoic cysts. (c and d) T2-weighted axial and coronal MR images show multiple tiny high-signal intensity renal cysts and low-signal intensity angiomyolipomas in both kidneys
24.4.5 Wilms Tumor
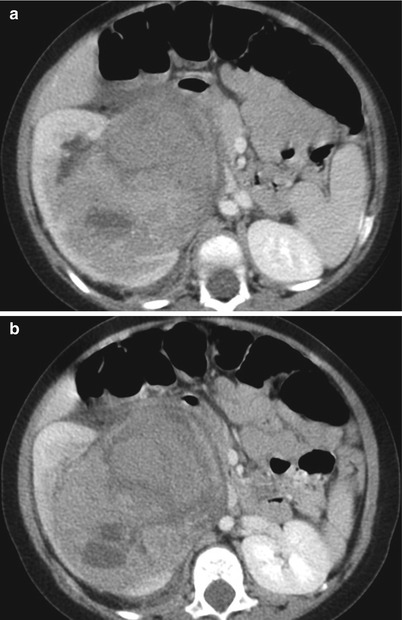
Fig. 24.8
Wilms tumor in a 1-year-old girl. (a and b) Contrast-enhanced CT scans show a heterogeneous mass arising from the renal sinus of the right kidney. The mass shows heterogeneous enhancement and irregular central necrosis and hemorrhage. The mass involves the renal sinus
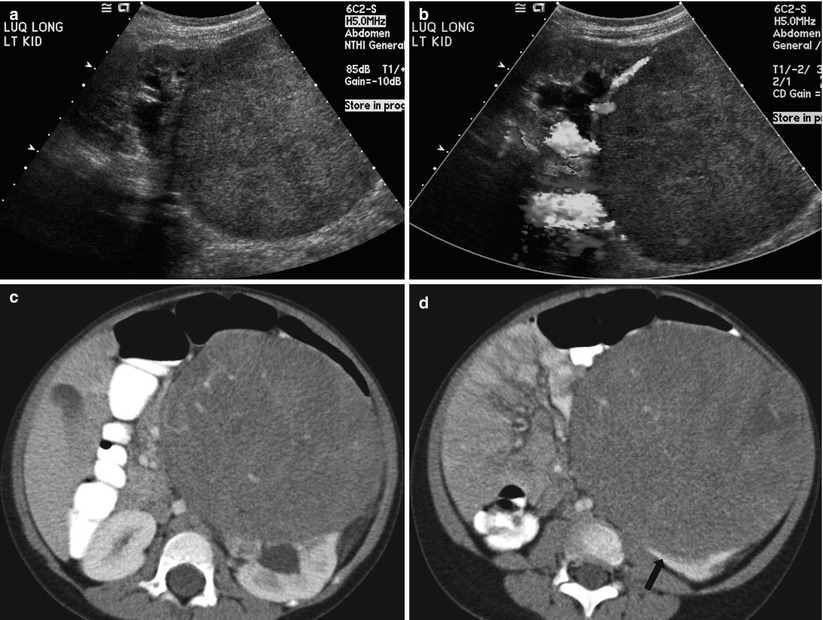
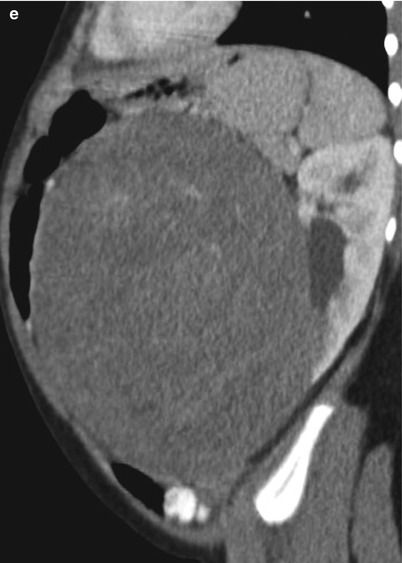
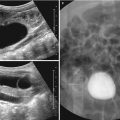
Fig. 24.9
Wilms tumor in a 3-year-old girl. (a) Longitudinal US of the left kidney shows a homogeneously echogenic mass arising from the lower pole of the kidney. Note the dilated renal pelvis of the left kidney. (b) Color Doppler US of the mass shows an intratumoral vascular structure. Note that tumor vascularity is far less than that of the normal renal parenchyma. (c, d, and e) Contrast-enhanced CT shows a large, low-attenuated, solid mass arising from the lower pole of the left kidney. The finding of a peripheral crescentic rim (arrow) of renal tissue surrounding a well-defined mass is helpful for confirming the renal origin of the tumor
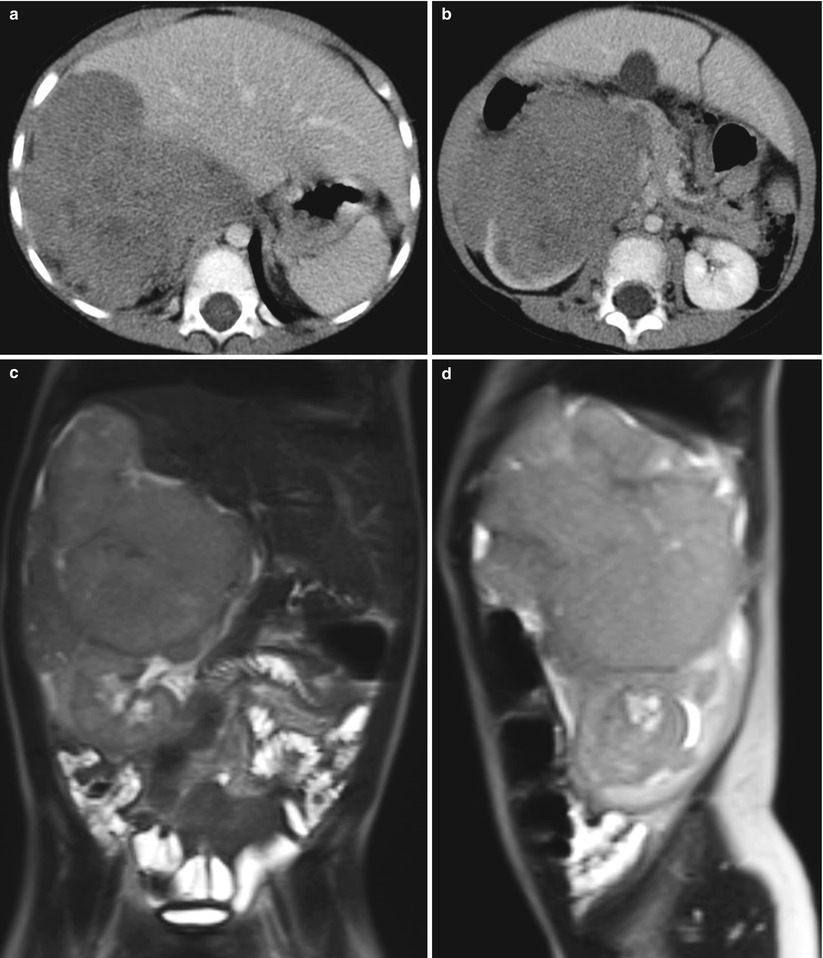
Fig. 24.10
Wilms tumor in a 2-year-old boy. (a and b) Contrast-enhanced CT scans show a large enhancing mass arising from the right kidney and extending into the right subhepatic and perirenal spaces. (c and d) T2-weighted axial MR images demonstrate a large heterogeneous signal intensity mass arising from the right kidney. The mass extends into the right subhepatic and perirenal space suggesting tumor rupture, which is confirmed in surgery
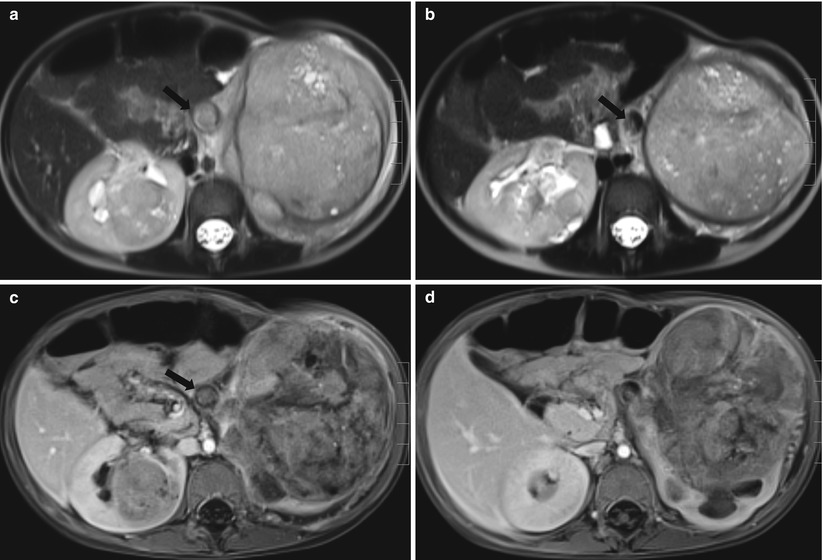
Fig. 24.11
Bilateral Wilms tumor in a 2-year-old boy with Drash syndrome. (a and b) T2-weighted axial MR images demonstrate heterogeneous signal intensity masses in both kidneys. The masses contain internal multiple tiny high-signal intensity lesions within the tumor, which represent necrotic areas. The left renal vein is distended and is filled with tumor thrombi (arrows). (c and d) Contrast-enhanced T1-weighted axial MR images demonstrate a large, heterogeneously enhanced mass arising from the left kidney and a small homogeneously enhanced mass in the right kidney. Note tumor thrombi in the left renal vein (arrow)
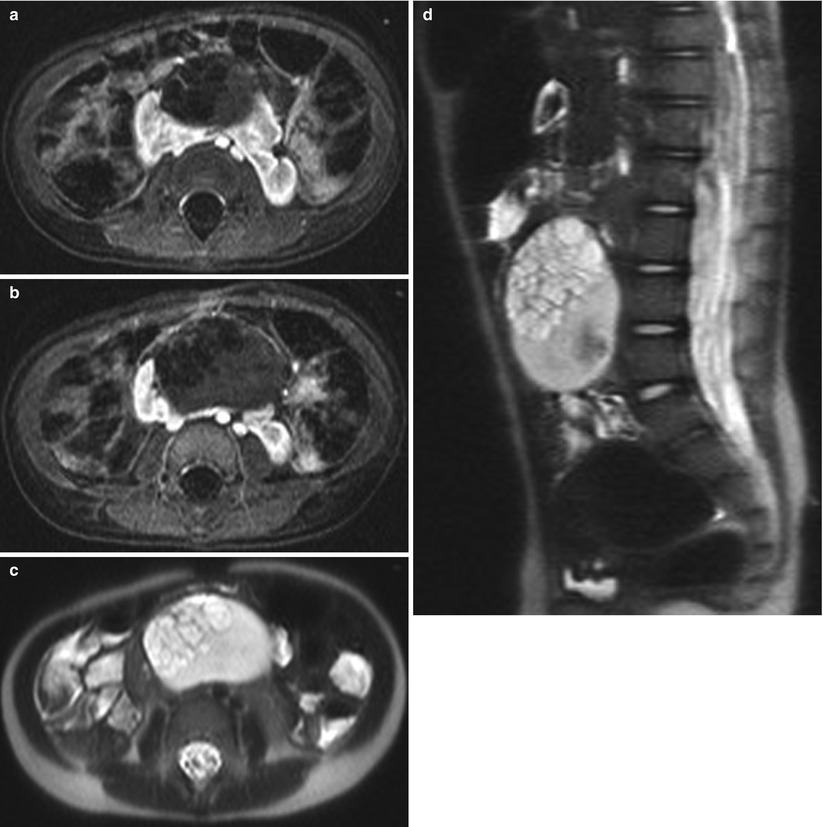
Fig. 24.12




Cystic Wilms tumor in a horseshoe kidney in a 2-year-old girl. (a and b) Contrast-enhanced T1-weighted axial MR images demonstrate a multiseptated, cystic, and partially solid mass arising from the isthmus of the horseshoe kidney. T2-weighted axial (c) and sagittal (d) MR images demonstrate a heterogeneous large multiseptated cystic mass
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree