, Joungho Han2, Man Pyo Chung3 and Yeon Joo Jeong4
(1)
Department of Radiology Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South Korea)
(2)
Department of Pathology Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South Korea)
(3)
Department of Medicine Division of Pulmonary and Critical Care Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South Korea)
(4)
Department of Radiology, Pusan National University Hospital, Busan, Korea, Republic of (South Korea)
Abstract
Mosaic attenuation pattern appears as patchwork of regions of differing attenuation that may represent (a) patchy interstitial disease, (b) obliterative small airway disease, or (c) occlusive vascular disease [1, 2]. Mosaic attenuation pattern caused by the latter two disease categories is called mosaic perfusion (Figs. 13.1 and 13.2). The combination of mixed lung attenuations (combination of ground-glass opacity, normal lung, and reduced lung attenuation as a result of mosaic perfusion) often gives the lung a geographic appearance and has been termed the head–cheese sign.
Mosaic Attenuation
Definition
Mosaic attenuation pattern appears as patchwork of regions of differing attenuation that may represent (a) patchy interstitial disease, (b) obliterative small airway disease, or (c) occlusive vascular disease [1, 2]. Mosaic attenuation pattern caused by the latter two disease categories is called mosaic perfusion (Figs. 13.1 and 13.2). The combination of mixed lung attenuations (combination of ground-glass opacity, normal lung, and reduced lung attenuation as a result of mosaic perfusion) often gives the lung a geographic appearance and has been termed the head–cheese sign.
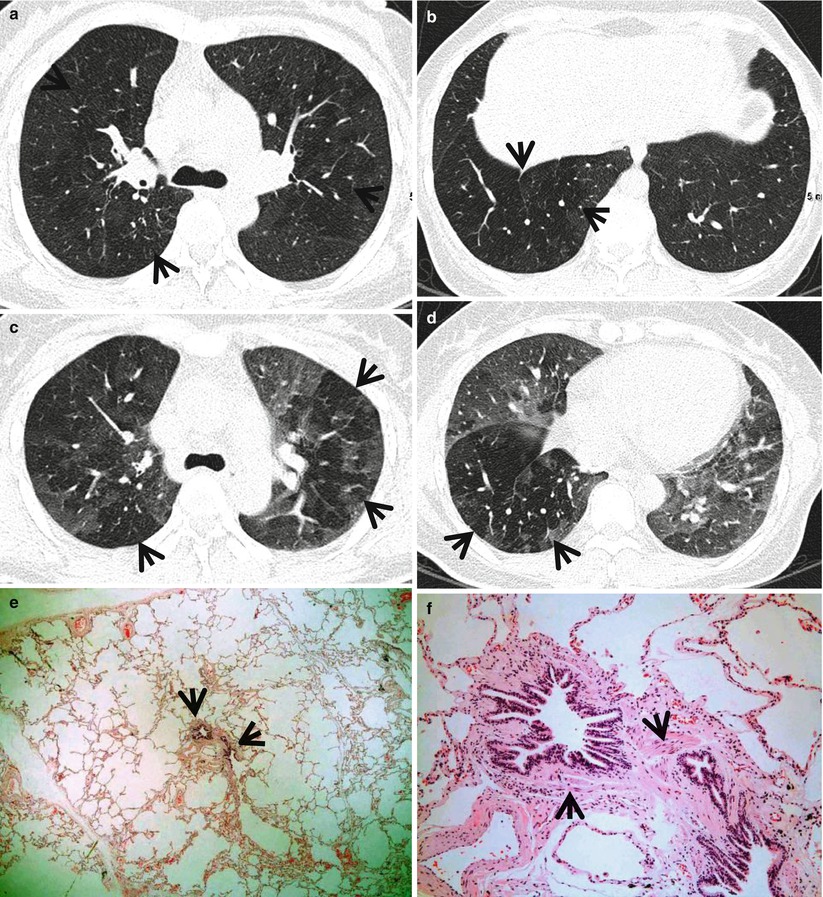
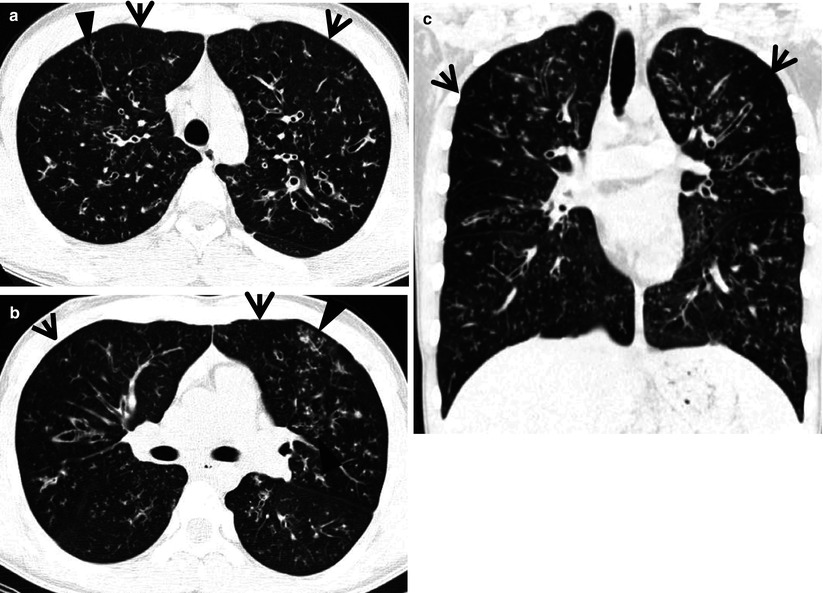
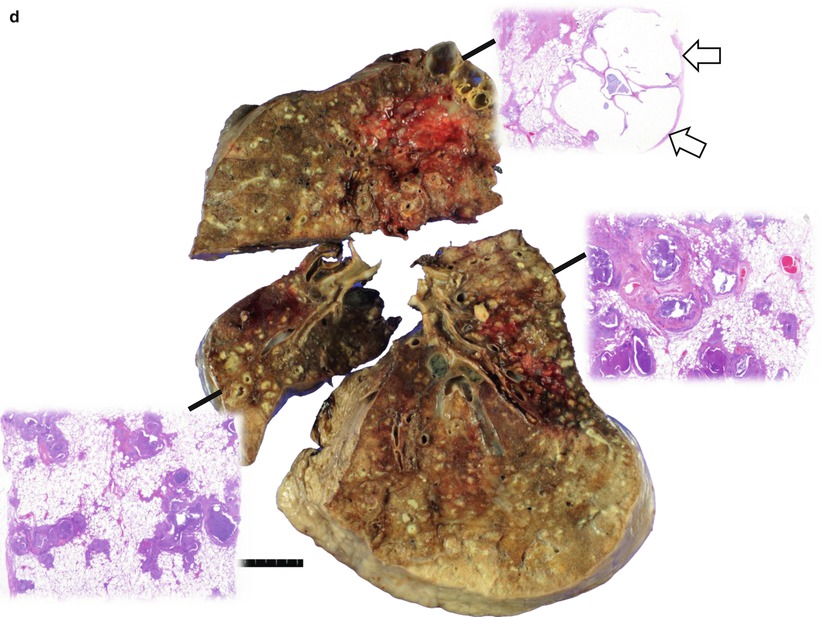

Fig. 13.1
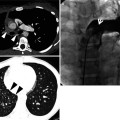
Constrictive bronchiolitis in a 45-year-old woman. (a, b) Lung window images of thin-section (1.5-mm section thickness) CT scans obtained at levels of main bronchi (a) and liver dome (b), respectively, show patchy areas of mosaic perfusion (arrows) in both lungs. (c, d) Expiratory CT scans obtained at similar levels to (a, c) and (b, d), respectively, demonstrate air trapping (arrows) more clearly in both lungs. (e) Low-magnification (×40) photomicrograph of pathologic specimen obtained with surgical lung biopsy displays bronchiolar collagen-type fibrosis inducing luminal narrowing (arrows) of a membranous bronchiole. (f) High-magnification photomicrograph (×200) discloses fibrous thickening of lamina propria between epithelium and muscularis mucosa (arrows)


Fig. 13.2
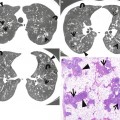
Cystic fibrosis in a 24-year-old man who underwent lung transplantation. (a, b) Lung window images of thin-section (2.5-mm section thickness) CT scans obtained at levels of aortic arch (a) and main bronchi (b), respectively, show extensive areas of bronchiectasis and cellular bronchiolitis (arrowheads) in both lungs. Also note bilateral patchy areas of mosaic attenuation (arrows). (c) Coronal reformatted CT image (2.0-mm section thickness) demonstrates bronchiectasis and cellular bronchiolitis in both lungs. Also note patchy areas of mosaic attenuation (arrows), in which oligemia (decreased caliber of vessels) findings are clearly visualized. (d) Gross pathology and photomicrographs at corresponding area indicated by bars of explanted lungs disclose pus in bronchiectatic or bronchiolectatic airways. In a portion of right lung, airway wall fibrosis and resultant postobstructive airway dilatation are seen (open arrows)
Diseases Causing the Mosaic Attenuation Pattern
Causes of mosaic attenuation pattern include infiltrative lung disease, airway disease, and vascular disease. Mosaic attenuation can be seen in a variety of airway diseases including bronchiectasis, cystic fibrosis (Fig. 13.2), allergic bronchopulmonary aspergillosis (ABPA), asthma, and constrictive bronchiolitis (Fig. 13.1). Vascular causes of mosaic perfusion include chronic pulmonary thromboembolism (Fig. 13.3) and pulmonary arterial hypertension (Fig. 13.4) (Table 13.1). Various interstitial lung diseases characterized by patchy areas of ground-glass opacity (GGO) are also the causes of mosaic attenuation pattern. Please note Chaps. 20 and 21 areas of GGO with or without reticulation. Mixed infiltrative and obstructive diseases (hypersensitive pneumonitis, sarcoidosis, atypical infection with associated bronchiolitis) also cause mosaic attenuation pattern.
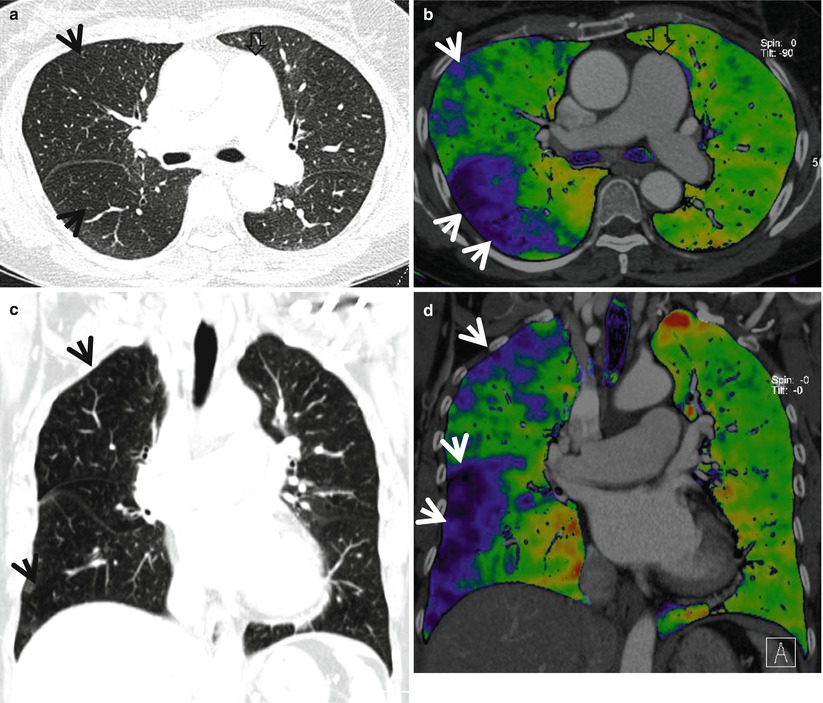
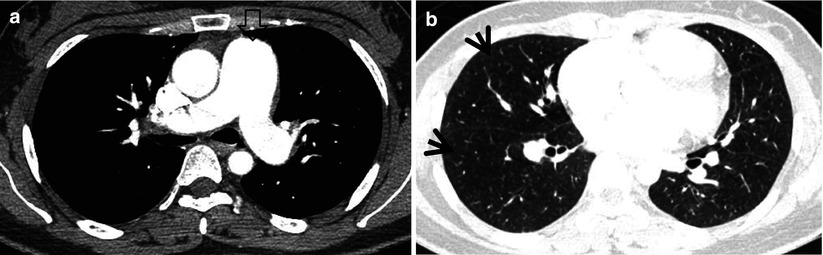

Fig. 13.3
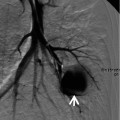
Mosaic perfusion in a 58-year-old woman with chronic thromboembolism and pulmonary arterial hypertension. (a) Lung window image of CT scan (1.0-mm section thickness) obtained at level of main bronchi shows mosaic attenuation areas (arrows) in right lung. Also note markedly enlarged main pulmonary artery. (b) Iodine perfusion map obtained with dual-source dual-energy CT clearly demonstrates areas of mosaic perfusion (arrows, blue-colored areas). (c) Coronal reformatted image (2.0-mm section thickness) shows multifocal areas (arrows) of mosaic attenuation. (d) Iodine perfusion map depicts more clearly mosaic perfusion areas (arrows)

Fig. 13.4
Mosaic perfusion in a 28-year-old woman with idiopathic pulmonary arterial hypertension. (a) Mediastinal window image of enhanced CT (1.0-mm section thickness) scan obtained at level of main bronchi shows markedly enlarged main pulmonary artery (open arrow). (b) Lung window image obtained at level of right inferior pulmonary vein demonstrates suspicious areas of mosaic attenuation (arrows)
Table 13.1
Common diseases manifesting as mosaic attenuation
Disease | Key points for differential diagnosis |
|---|---|
Airway disease | |
Bronchiectasis | Bronchial dilatation with peribronchial thickening, signet ring sign |
Cystic fibrosis | Mosaic perfusion, bronchiectasis involving upper lobes |
ABPA | Central bronchiectasis with high-attenuation mucus plugging, mosaic perfusion |
Asthma | Thickening and narrowing of the medium-sized and small bronchi, cylindrical bronchiectasis, centrilobular nodules, multifocal and patchy areas of mosaic perfusion |
Constrictive bronchiolitis | Mosaic perfusion, air trapping on expiratory scans |
Vascular disease | |
Chronic pulmonary thromboembolism | Mosaic perfusion, ipsilateral airway dilatation, vascular findings of chronic embolism |
Pulmonary arterial hypertension | Mosaic perfusion, central pulmonary arterial dilatation in the absence of detectable intraluminal thrombi |
Mixed infiltrative and obstructive disease | |
Subacute hypersensitivity pneumonitis | Combination of GGO on inspiratory scan and air trapping on expiratory scan |
Sarcoidosis | Associated with perilymphatic micronodules |
Atypical pneumonia with bronchiolitis | Patchy areas of GGO and centrilobular nodules, mosaic perfusion |
Distribution
In cystic fibrosis, proximal or perihilar bronchi are always involved when bronchiectasis is present. All lobes are typically involved, although early in the disease, abnormalities show often predominantly upper lobe predominance in their distribution [3]. Although there are some overlaps, areas of mosaic perfusion in cystic fibrosis correspond to pulmonary lobules or subsegments [4], whereas those in chronic thromboembolism are typically segmental or subsegmental in distribution [5]. In subacute hypersensitivity pneumonitis, areas of GGO are usually diffuse, bilateral, and symmetric. However, areas of mosaic perfusion are multifocal and usually have a configuration consistent with involvement of single or multiple adjacent pulmonary lobules [6].
Clinical Considerations
Cystic fibrosis results from an autosomal-recessive genetic defect in the structure of the cystic fibrosis transmembrane regulator protein, which leads to abnormal chloride transport across epithelial membranes [7]. ABPA results from both type I and type III hypersensitivity reactions to the endobronchial growth of Aspergillus species and is characteristically associated with eosinophilia, symptoms of asthma, and typical imaging findings [8]. Conditions associated with constrictive bronchiolitis include heart–lung or lung transplantation, chronic allograft rejection, allogeneic bone marrow transplantation with chronic graft-versus-host disease, and collagen vascular disease, especially rheumatoid arthritis [9]. Pulmonary arterial hypertension may be idiopathic or arise in association with chronic pulmonary thromboembolism; pulmonary embolism caused by tumor cells, parasitic material, or foreign material; parenchymal lung disease; liver disease; vasculitis; human immunodeficiency virus infection; or a left-to-right cardiac shunt [5]. Most cases of hypersensitivity pneumonitis develop only after many years of inhaling allergens, which include microbes, animal or plant proteins, and certain chemicals [10].
Key Points for Differential Diagnosis
1.
Regardless of its cause, when mosaic perfusion is present, pulmonary vessels in the areas of decreased opacity often appear smaller than vessels in relatively dense areas of the lung [11, 12]. This discrepancy can be quite helpful in distinguishing mosaic perfusion from patchy appearance of GGO. In patients with GGO, vessels usually appear equal in size throughout the lungs.
2.
In patients with mosaic perfusion resulting from airway diseases, abnormally dilated or thick-walled airways may be visible in the relatively lucent lung regions [2], and lobular areas of low attenuation are common [13]. Air trapping on expiratory scans is often helpful in confirming the diagnosis. Attenuation differences are accentuated scans obtained on expiration [14].
3.
In patients with mosaic perfusion resulting from vascular diseases, areas of low attenuation are usually larger than lobules. In patients with mosaic perfusion occurring in association with chronic pulmonary embolism or pulmonary arterial hypertension, enlargement of the main pulmonary arteries may be visible.
4.
Characteristic CT vascular signs including webs or bands, intimal irregularities, abrupt narrowing, or complete obstruction of the pulmonary arteries with enlarged main pulmonary arteries enable the diagnosis of chronic pulmonary thromboembolism in patients with mosaic perfusion [15].
5.
Data from electrocardiographically (ECG)-gated multidetector CT studies show that functional parameters such as right pulmonary artery distensibility, systolic–diastolic right ventricular outflow tract dimensions, and diastolic wall thickness can be measured with good interobserver agreement and used as reliable criteria for a diagnosis of pulmonary hypertension [16]. Mosaic lung perfusion is found significantly more often among those with pulmonary arterial hypertension due to vascular disease than among those with pulmonary arterial hypertension due to cardiac or lung disease (74 % [17 of 23] vs. 8 % [3 of 38] of the patients in one series) [17].
6.
The head-cheese sign is usually indicative of mixed infiltrative and obstructive disease, usually associated with bronchiolitis. In patients with this appearance, the presence of GGO is caused by lung infiltration, whereas the presence of mosaic perfusion with decreased vessels sign is usually caused by small airway disease. The combination of GGO on inspiratory scans and air trapping on expiratory scans is considered indicative of a mixed infiltrative and obstructive disease such as hypersensitivity pneumonitis [14].
Cystic Fibrosis
Pathology and Pathogenesis
Cystic fibrosis is an autosomal-recessive disease with a mutation of cystic fibrosis transmembrane conductance regulator (CFTR) gene and multisystem involvement. Patients have abnormal transport of chloride and sodium across the respiratory epithelium, resulting in thickened airway secretions and susceptibility to recurrent infections. Grossly, widespread bronchiectasis (more severe in upper lobe) with thick mucus plugs, pleural fibrosis or adhesions, pneumonic consolidation, and lobar atelectasis are seen in an end-stage disease (Fig. 13.2). Microscopically, acute and chronic inflammations involving the large and small airways associated with bronchial gland and goblet cell hyperplasia, squamous metaplasia, and mucostasis are frequently seen (Fig. 13.2).
Symptoms and Signs
Since cystic fibrosis is a genetic disease resulting in complications in multiple organs, especially involving the lungs and pancreas, it mimics a number of other diseases. Usual respiratory presentations in adults include cough, sputum, wheezing, dyspnea, recurrent respiratory tract infection, and cor pulmonale if advanced. Usual gastrointestinal presentations in adults are recurrent abdominal pain, biliary cirrhosis with portal hypertension, and recurrent pancreatitis. Infertility may occur.
CT Findings
The predominant HRCT finding in early stage of cystic fibrosis is a mosaic perfusion due to air trapping related to small airway disease. Other typical CT features include bronchiectasis and peribronchial thickening, mainly involving the upper lobes, and centrilobular nodules or a tree-in-bud pattern and atelectasis or consolidation secondary to mucous plugging [3, 18] (Fig. 13.2). Bronchiectasis is most often cylindrical, but varicose and cystic bronchiectasis can be seen in advanced cases.
CT–Pathology Comparisons
Cystic fibrosis causes abnormal mucous gland secretions and the subsequent effect of inflammation and infection on airways [3]. The earliest and most universal pathologic lesion of cystic fibrosis is mucous obstruction of bronchioles and small bronchi (Fig. 13.2). A mosaic perfusion, which is the predominant early HRCT findings of cystic fibrosis, is caused by air trapping related to mucous obstruction and inflammation of bronchioles and small bronchi (Fig. 13.2). The elicited inflammatory response damages the normal structure of the airways, and bronchiectasis develops. Upper lobe predominance of bronchiectasis and peribronchial thickening may reflect an effect of gravity on the elastin-damaged parenchymal tissue. Obstruction of airways by mucous plugs results in centrilobular small nodules or a tree-in-bud pattern and atelectasis or consolidation on HRCT.
Patient Prognosis
Treatment consists of control of mucus retention and chronic infection in the lungs, replacement of pancreatic enzymes, and nutritional therapy. New therapeutic approaches, including pharmacologic interventions and gene transfer, offer hope for further advances [19]. Lung transplantation has become an accepted therapy for respiratory failure secondary to cystic fibrosis. The outcome is highly variable, but 50 % of patients can now be expected to survive beyond 37 years.
Constrictive Bronchiolitis
Pathology and Pathogenesis
Constrictive bronchiolitis is a small airway disease in which variable narrowing or obliteration of airway lumens occurs. Histologically, the disease shows submucosal scarring, concentric luminal narrowing, adventitial scarring, and chronic inflammation. In the late stage, the lumen is completely occluded by the fibrosis (Fig. 13.1).
Symptoms and Signs
Clinically, constrictive bronchiolitis is characterized by progressive airflow obstruction with poor responsiveness to medical therapy and high mortality rates [20]. Patients complain of cough and dyspnea, which progress relentlessly over weeks to months. Physical examination reveals inspiratory squeaks in 40–60 % of patients.
CT Findings
The main HRCT findings usually consist of areas of mosaic perfusion associated with vessels of decreased caliber on inspiratory scans and air trapping on expiratory scans [21]. Air trapping on expiratory HRCT is the most sensitive sign to detect constrictive bronchiolitis on HRCT [22] (Fig. 13.1). Central and peripheral bronchiectasis is also commonly present. Other findings include centrilobular small nodules and tree-in-bud patterns.
CT–Pathology Comparisons
Mosaic perfusion on HRCT is presumably caused by hypoventilation of the alveoli distal to the bronchial obstruction, which leads to secondary vasoconstriction. This vasoconstriction can be seen on CT scans as areas of decreased attenuation [23] (Fig. 13.1). Bronchiectasis may be secondary to the constrictive bronchiolitis itself or to a prior insult to the bronchial wall. Centrilobular small nodules and tree-in-bud patterns on HRCT are caused by peribronchiolar thickening and bronchiolectasis with secretions [24].
Patient Prognosis
Prognosis of constrictive bronchiolitis, irrespective of etiology, is poor. Most patients progressively deteriorate and are ultimately fatal due to respiratory failure within months to years. Macrolide antibiotics exhibit immunomodulatory effects and have been used.
Chronic Pulmonary Thromboembolism
Pathology and Pathogenesis
Chronic pulmonary thromboembolism (PE) causes pulmonary hypertension secondary to obstruction of arteries due to organized thrombi. In the lung of chronic PE, distended capillaries, multifocal microscopic foci of alveolar wall necrosis with focal inflammation, small amount of fibrinous exudate, and edema fluid can be seen. Variable amount of bronchopneumonia may be accompanied.
Symptoms and Signs
Patients with chronic PE typically present with nonspecific symptoms occurring in those with other causes of pulmonary hypertension [25]. The symptoms include progressive dyspnea on exertion, chronic fatigue, chest discomfort, palpitations, or syncope. Cardiac murmur may be heard. Lower extremity edema, ascites, hepatomegaly, and cyanosis can be found.
CT Findings
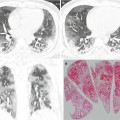
The most common pulmonary parenchymal finding of chronic PE is a mosaic perfusion pattern (combination of areas of decreased attenuation and perfusion adjacent to areas of increased attenuation and perfusion) [15] (Fig. 13.3). Mosaic perfusion patterns are typically distributed in segmental and subsegmental patterns [26]. Chronic PE may also be associated with ipsilateral airway dilatation [27]. Vascular findings of chronic PE include webs or bands, intimal irregularities, abrupt narrowing or complete obstruction of pulmonary arteries, and enlargement of main pulmonary artery [15].
CT–Pathology Comparisons
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree