Venous thromboembolism (VTE) is an important worldwide clinical entity. It includes asymptomatic deep venous thrombosis (DVT), symptomatic DVT, and pulmonary embolism (PE). Each year, DVT and PE affect 1 to 2 per 1000 people in the United States. In persons more than 80 years old, the incidence of DVT increases to 1 in 100. One third of the people who had DVT may go on to develop long-term complications (e.g., postthrombotic syndrome) with symptoms of swelling, pain, and discoloration of the affected limb. Recurrent DVT occurs in one third of patients within 10 years of the initial diagnosis. Approximately 60,000 to 100,000 people die of DVT or PE each year in the United States.
Diagnostic Methods
Clinical and ancillary findings are useful for the diagnosis of DVT. Clinical scoring systems such as the Wells score can be used to select patients for an appropriate diagnostic test. Noninvasive tests, such as the D-dimer test, and plethysmography may be used for diagnosis of DVT. However, sensitivity varies, and specificity is poor ( Table 49-1 ). The definitive diagnosis depends on identifying the presence, location, and extent of the thrombus on imaging studies. Ultrasonography and color Doppler imaging are commonly used; computed tomography venography (CTV) and magnetic resonance venography (MRV) play significant roles in identifying pelvic, abdominal, and chest DVT. Ascending venography has little role in the diagnosis of DVT, although it is used in therapeutic interventions (see Table 49-1 ).
| Test | Advantages | Disadvantages |
|---|---|---|
| D-dimer test | Rapid Inexpensive | Variable sensitivity Poor specificity in certain patient populations |
| Ultrasound | Relatively inexpensive Can be performed at bedside Highly sensitive for above-knee symptomatic DVT | Operator dependent Poor specificity for calf vein and pelvic vein DVT |
| CT venography | Highly sensitive and specific for central and pelvic vein DVT Can be used in patients with inherent contraindications for MRI | Expensive Radiation exposure Adverse reactions to contrast material |
| MRV | Highly sensitive and specific for central and pelvic vein DVT Can be used in patients allergic to iodinated contrast material Both noncontrast and gadolinium-enhanced studies possible | Expensive Poor availability Cannot be used in patients with pacemakers or aneurysm clips and in those who are claustrophobic Gadolinium contraindicated in patients with renal failure |
| Ascending venography | Highly sensitive and specific for DVT, including calf vein DVT | Invasive Poor availability Radiation exposure Adverse reactions to contrast material Risk of thrombophlebitis and contrast material extravasation |
Ultrasonography
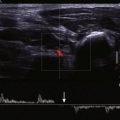
Ultrasonography is the imaging test of choice for assessment of DVT in the extremities. This technique is recommended for symptomatic patients in whom the suspicion of acute DVT is high. Grayscale ultrasound is coupled with Doppler (duplex ultrasound) imaging for an accurate assessment of the venous system. This approach has greater than 95% sensitivity and specificity for detecting above-knee DVT.
Technique
The deep and superficial veins of the extremities are usually examined from proximal to distal locations (i.e., from the common femoral vein to the tibial veins or from the subclavian vein to the forearm veins). At each location, the vein is compressed directly with the ultrasound probe to assess compressibility. In addition, Doppler evaluation of the vein is performed (either with or without color flow imaging) to assess presence of flow changes to respiration and cardiac motion and the response to distal compression. Normal veins are compressible and demonstrate an anechoic lumen ( Fig. 49-1 ). The venous flow is phasic with respiration and shows an augmentation response to distal compression ( Fig. 49-2 ). Upper extremity veins often demonstrate flow variations secondary to cardiac pulsations. This phenomenon is often observed in the jugular, subclavian, and axillary veins. Deep inspiration may collapse a normal subclavian or jugular vein.


Findings
An enlarged vein that is noncompressible and demonstrates low-level echoes within the lumen is diagnostic of acute DVT ( Fig. 49-3 , A ). Color flow and Doppler imaging techniques demonstrate no flow within the lumen (see Fig. 49-3 , B ). The response to distal compression is blunted. Partial thrombosis of the vein manifests as a partially compressible vein with partial flow noted on color flow imaging ( Fig. 49-4 ). Subacute thrombosis is depicted as a normal-caliber vein that is noncompressible or partially compressible with a moderately echogenic lumen and an absence of color flow or Doppler signal. Partial recanalization of the thrombus results in the appearance of flow channels within the thrombus; these channels are best observed on color flow or power Doppler imaging ( Fig. 49-5 ). As the thrombosis becomes chronic, the caliber of the vein becomes small, with little or no flow. Calcification of the venous wall indicates chronic DVT and may be seen as a highly echogenic focus with or without distal acoustic shadowing.



Upper extremity DVT has similar imaging characteristics. The absence of normal cardiac pulsations and of a response to deep inspiration may suggest the presence of DVT in the proximal veins.
Pearls and Pitfalls
- ▪
Ultrasound may identify other causes of leg pain, such as a ruptured or unruptured Baker cyst, hematoma, and tendon injury.
- ▪
Visualizing the intrathoracic and abdominopelvic veins is often difficult. Proximal (or central) venous compression or thrombosis may be suspected when respiratory phasic variations in the distal veins are absent (see the later section on May-Thurner syndrome).
- ▪
When DVT is detected, the examiner must assess its proximal extent because pelvic DVT is more often associated with PE.
- ▪
The sensitivity of duplex ultrasound in detecting symptomatic DVT is greater than 95%; however, it falls to 50% for detection of asymptomatic DVT. When the ultrasound study result is negative in patients with a high clinical suspicion of DVT, a repeat ultrasound study 1 week later is recommended.
Computed Tomography Venography
CTV allows visualization of central thoracic and abdominopelvic veins that are often obscured on ultrasound. One of the main advantages of CTV is that the study can be combined with computed tomography pulmonary angiography (CTPA) without the requirement of additional intravenous contrast material. These protocols allow 20% higher detection of VTE compared with CTPA alone. The extent of the computed tomography (CT) scan ranges from the popliteal fossa to the groin or the pelvis. The scan may be extended to the upper abdomen if thrombosis of the inferior vena cava (IVC) or gonadal or renal veins is suspected.
Technique
CTV may be performed following intravenous injection of diluted (10%) iodinated contrast material in the foot veins or upper extremity veins to assess regional venous thrombosis. This technique is referred to as direct CTV and is rarely practiced now, but it can be useful when other studies are indeterminate. Indirect CTV refers to CT assessment of the regional and nonregional veins following intravenous administration of regular-strength contrast material. The veins are imaged during equilibrium phase (approximately 3 minutes following the injection of contrast material). Images are reconstructed at 5- to 7.5-mm slice thickness with no interslice gap. Venous enhancement is considered adequate when the attenuation in the vein is higher than in the adjacent muscles; the enhanced veins rarely achieve an attenuation of more than 150 HU.
Findings
CTV allows direct visualization of thrombus in the vein as a filling defect within the contrast-filled lumen ( Fig. 49-6 ). When thrombus fills the entire lumen and extends for a few CT slices, that segment of the vein fails to enhance with contrast material. Additional findings of acute thrombosis include an enlarged vein, rim enhancement of the vein (see Fig. 49-6 ), and perivenous edema or stranding. The sensitivity and specificity of CTV in detecting above-knee DVT are 90% to 95% compared with those of venous ultrasonography. Chronic thrombosis may be seen as nonenhancing small-caliber vein or intraluminal webs with enhancement of recanalized channels; the presence of calcification within the venous wall is diagnostic of chronic DVT ( Fig. 49-7 ).


Pearls and Pitfalls
- ▪
Excellent visualization of the central and peripheral veins makes CTV an optimal test for the diagnosis of DVT.
- ▪
CTV allows detection of other leg disorders such as popliteal cysts, iliopectineal bursitis, joint effusions, and intramuscular hemorrhage.
- ▪
Radiation exposure can be minimized by limiting the study to the thighs and scanning at lower peak kilovoltage.
- ▪
Inadequate enhancement of the veins can be minimized by using autotriggered scanning while keeping a region of interest in the femoral vein with a threshold of 60 HU. Larger volume of contrast material, low peak kilovoltage (80 kVp), and reconstruction at 50 keV on a dual energy scanner allow better visualization of the veins on indirect CTV.
- ▪
Image visualization at narrow window length and coronal reformations are also helpful to delineate the thrombus and its longitudinal extent.
Magnetic Resonance Venography
MRV is less commonly used for detection of lower extremity DVT. It is often used to assess the central veins of the thorax and body. It is equally sensitive and specific for detection of DVT in both the central and peripheral veins. MRV can be performed either with or without the use of gadolinium. Noncontrast MRV methods include bright-blood techniques such as time of flight (TOF) imaging, true fast imaging with steady-state precession (true FISP), and electrocardiogram-gated three-dimensional fast spin echo (FSE) imaging and black-blood techniques such as FSE T2-weighted and double inversion recovery FSE imaging. Contrast-enhanced MRV techniques use three-dimensional gradient echo techniques similar to magnetic resonance angiography (MRA) for venous phase imaging. Rarely, direct MRV (similar to direct CTV) is performed with diluted (2%) gadolinium. Blood pool contrast materials (e.g., gadofosveset trisodium) are best suited for venous imaging.
Technique
The axial two-dimensional TOF technique is employed for abdominopelvic and lower extremity veins, with a saturation band applied superiorly to prevent artifacts from the arterial inflow. True FISP imaging is usually performed in the coronal plane for the body and in the axial plane for extremities. Images are acquired in 3- to 5-mm slice thickness. Contrast-enhanced MRV is usually performed in the coronal plane.
Findings
On bright-blood techniques and contrast-enhanced MRV, thrombus is seen as a filling defect within the vein ( Fig. 49-8 ). Long segment thrombosis is best appreciated on coronal or sagittal images and is visualized as discontinuity of the vessel. However, the venous wall may enhance on contrast-enhanced MRV, thus giving a tram-track appearance ( Fig. 49-9 ). Perivenous edema is rarely appreciated, but it can be seen on T2-weighted FSE sequences. On black-blood techniques, thrombus is seen as a mass of low signal intensity within the lumen of the vein. Chronic thrombus is seen as a small-caliber, nonenhancing vein with or without venous stenosis. Collateral veins are often seen bypassing the obstructed vein.


Pearls and Pitfalls
- ▪
Flow directional abnormalities may result in signal void on TOF sequences. Additional imaging either with true FISP or contrast-enhanced MRV may be required to limit false positive diagnosis of DVT. On the other hand, the flow directional nature of TOF sequence can be utilized for advantage while assessing the hemodynamic significance of venous stenoses as collateral veins may show flow directional abnormalities (see the later section on May-Thurner Syndrome).
- ▪
True FISP imaging can be combined with background suppression to provide better contrast resolution of the veins. During contrast-enhanced MRV, delayed (2 minutes), axial fat-saturated gradient echo, high-resolution T1-weighted images allow assessment of partial and wall-adherent thrombus.
- ▪
Similar to CTPA, MRV can be combined with pulmonary MRA, especially with the use of blood pool contrast materials.
Ascending Venography
Although ascending venography remains the gold standard, it is rarely performed for the diagnosis of DVT because it has been replaced by ultrasonography for extremity DVT. Ascending venography has 95% specificity and 100% sensitivity for thrombi measuring 0.5 cm or more, and it is still the test of choice for detection of below-knee DVT. It is used in interventional procedures such as thrombolysis, placement of an IVC filter, and revascularization of venous occlusion.
Technique
Upper extremity venography is performed with the patient supine, with hands abducted and supinated. A superficial vein in the dorsum of the hand is accessed with an 18-gauge needle. A blood pressure cuff that is inflated to nearly systolic pressure or a tourniquet is applied in the upper arm, and diluted (240 mg iodine/mL) contrast material is injected. Multiple overlapping fluoroscopic images are obtained for the forearm and arm. The central veins are imaged while the contrast material is injected and the blood pressure cuff is deflated. Proximal venous access is often helpful for visualizing the central veins.
Lower extremity venography is best performed with the patient in the reverse Trendelenburg position at 30 to 60 degrees. The limb examined is kept nonweight bearing, and a dorsal foot vein is cannulated with an 18-gauge needle. A tourniquet applied at the ankle helps direct contrast material in to the deep venous system. Diluted contrast material (240 mg iodine/mL) is injected to a volume of 50 to 100 mL, and serial fluoroscopic images are obtained of the extremity. Pelvic veins are best imaged with the patient in the supine position.
Descending venography is performed for assessing venous insufficiency. In this procedure, a short sheath is placed in the common femoral vein, the patient is positioned at 60 degrees in the reverse Trendelenburg position, and 20 mL of contrast material is injected. Retrograde flow into the femoral and saphenous veins indicates the presence of venous reflux.
Findings
As with CT, acute thrombus is seen as a filling defect within the contrast-filled lumen ( Fig. 49-10 ). The vein may be enlarged, and collateral veins may be visualized. Chronic thrombosis results in nonvisualization of the vein, with multiple collateral veins bypassing the occluded vein. Recanalized veins may appear as linear channels within the expected course of a vein.