(1)Hypertension
(2)Atherosclerosis
(3)Thoracic aortic aneurysm
(4)Connective tissue disorders
Marfan syndrome
Loeys-Dietz syndrome
Ehlers-Danlos syndrome
Turner syndrome
(5)Bicuspid aortic valve
(6)Coarctation of the aorta
(7)Aortitis
(8)Iatrogenic
(9)Sheehan syndrome
(10)Cushing syndrome
(11)Hypervolemia (pregnancy)
(12)Polycystic kidney disease
(13)Pheochromocytoma
Common evolutive scenarios include:
Frank rupture of the false lumen throughout the adventitial wall
Impending rupture with peri-aortic hematoma
False lumen compression of the true lumen and/or origin of aortic branches (arch vessels, splanchnic and limb arteries) with malperfusion syndrome.
Chronic remodeling and/or dilatation of the dissected aorta
1.3 Classification
Two classification systems are most frequently used in clinical practice: the Stanford and the De Bakey systems (Fig. 16.1).
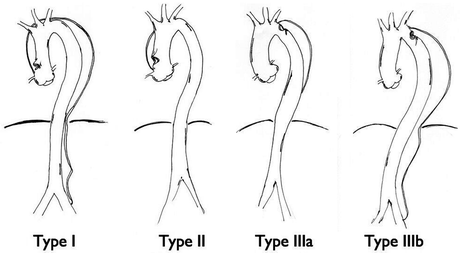

Fig. 16.1
De Bakey classification for acute aortic dissections
Stanford type A includes dissections that involve the ascending aorta whereas Stanford type B includes dissections that originate in the descending thoracic and thoracoabdominal aorta.
De Bakey type I involves the ascending aorta, the aortic arch and thoracoabdominal aorta; De Bakey type II only involves the ascending aorta; De Bakey type III involves the descending thoracic aorta (subtypes A) or the thoracoabdominal aorta (subtypes B).
A further subclassification is based on the timing of dissection.
1.
Acute dissection—presentation within the first 2 weeks.
2.
Subacute—presentation between 2 weeks and 2 months
3.
Chronic—presenting at greater than 2 months following the initial event.
The present chapter will focus on acute Stanford type B aortic dissection.
1.4 Diagnosis: Clinical
Clinical presentation is variable, from sudden death due to aortic rupture or more typically severe and unrelenting chest/back/abdominal pain. The character of the pain is often described as “ripping” or “tearing,” often migratory. Patients may also present with signs or symptoms related to malperfusion affecting the brain, spinal cord, limbs, or visceral organs. These findings may confuse the initial diagnosis. Tachycardia, anxiety, hypertension, or hypotension may accompany the clinical picture. Pulse differential or absence and/or unilateral (left) loss of breath sounds (hemothorax or inflammatory pleural effusion) represent frequent signs of type B aortic dissection.
1.5 Imaging
1.5.1 Chest X-ray
Mediastinal widening is a very sensitive X-ray finding despite its low specificity; the combination of chest pain, pulse differential and substantial mediastinal widening is highly (83%) predictive of acute dissection. Therefore, an accurate clinical evaluation may facilitate a prompt diagnosis leading to further imaging evaluation.
1.5.2 CT and MRI
Contrast-enhanced, cardiac-gated multidetector CT with sensitivity and specificity approaching 100% combined with rapidity of execution and wide availability in emergency departments represents the “gold standard” for evaluating acute dissection. MRI, although providing superior anatomical details than CT, is limited by availability and long examination time. High-resolution imaging modalities allow an accurate anatomic selection of candidates for endovascular treatment by delineating the intimal flap and its extension, the femoral access, the involvement of visceral branches, and the distance of the entry tear from arch vessels or the presence/absence of associated intramural hematoma required to identify an adequate proximal neck.
1.5.3 Echocardiography
Although an effective portable diagnostic tool, it does not sufficiently visualize the aortic arch or abdominal aorta or permit 3D reconstructions; nevertheless, its role is essential in guiding endovascular procedures.
1.5.4 Catheter Angiography
Is reserved only for patients in whom other modalities have been unable to identify the required anatomical details (rare) or during endovascular repair.
1.6 Management
Medical treatment in an intensive care unit is generally recommended for uncomplicated type B aortic dissections. It has been demonstrated that patients may survive their initial hospital stay with aggressive antihypertensive therapy, commonly intravenous labetalol to begin with, and keeping heart rate below 60 bpm significantly decreases secondary adverse events such as aortic expansion, recurrent aortic dissection and aortic rupture. As the aortic dissection is better controlled with anti-hypertensives, the pain shall also resolve.
Indication for endovascular or open surgical repair is appropriate in patients with acute type B aortic dissection presenting with:
1.
Signs of impending aortic rupture at clinical and imaging evaluation and/or
2.
Evidence of severely impaired visceral/peripheral perfusion. Results from the IRAD registry demonstrated that, in the setting of acute complicated type B dissection, the endovascular treatment provides lower hospital mortality and morbidity rates than open surgical repair.
Key goals for endovascular treatment for type B acute dissection are:
Sealing of the proximal tear
Depressurization and shrinkage of the false lumen leading to subsequent remodeling and stabilization of the aorta
Resolution of dynamic malperfusion without any adjunctive procedure