CHAPTER 1 Detection of Breast Cancer: Screening of Asymptomatic Patients
Breast cancer is the most common malignancy and the second leading cause of cancer deaths among American women. In 2005, it is estimated that more than 211,000 new cases will be diagnosed, and more than 40,000 women will die of the disease.1 Breast carcinoma mortality in the United States has declined substantially over the past 30 years, from 31.4 deaths per 100,000 women per year in 1975 to 25.9 deaths per 100,000 women per year in 2001.2 More recent analysis by the CDC of 1999–2003 data from the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) study and the CDC National Program of Cancer Registries (NPCR) indicated that age-adjusted incidence rates for invasive breast cancer decreased each year from 1999 to 2003, with the greatest decrease (6.1%) occurring from 2002 to 2003. For in situ cancers, rates increased each year from 1999 to 2002 and then decreased from 2002 to 2003, although the percentage decrease (2.7%) was smaller than that for invasive cancers (6.1%). In addition to advances in treatment options, the combination of increasing utilization of screening mammography and improved mammographic quality, allowing detection of cancers at an earlier stage, is likely to account for the reduction in breast cancer mortality.3–4
SCREENING MAMMOGRAPHY
To date, the benefits from screening mammography for women 40 to 70 years of age have been proven in eight randomized controlled trials (RCTs) conducted in Europe and the United States during the past 40 years.5–11 Reported reductions in breast cancer mortality range from 20% to 45%. Because of the relatively small numbers of women aged 40 to 49 years in the individual trials, the benefit from screening in this age range has been controversial. However, in 1997, a meta-analysis of women aged 40 to 49 years in all five Swedish trials found a 30% reduction in breast cancer deaths.12 In addition, long-term follow-up of three trials (Health Insurance Plan Project [HIP], Gothenburg, and Malmö) each found statistically significant reductions in breast cancer mortality.13–15 Therefore, for average risk women, most professional organizations in the United States recommend screening mammography beginning at the age of 40 years. For screening women younger than the age of 40 years, there are few RCT data. Therefore, decisions concerning screening practice in this age group must be based on less rigorous evidence and on a more individual basis. Given that younger women have a longer life expectancy and that cancers tend to grow more rapidly in younger women, earlier detection of these cancers may theoretically be advantageous. However, this must be balanced by the limitations of screening mammography in younger women, which include a lower frequency of breast cancer, reduced sensitivity of mammography, slightly increased radiation risk, and higher recall rates. Screening of younger women will be of most benefit in women at high risk, especially those known to carry the BRCA1 or BRCA2 gene mutation. Methods for estimating risk based on medical and family history include the Gail, Claus, and BRCAPRO mathematical models. Observational studies of high-risk women aged 30 to 39 years show a cancer detection rate similar to that for women aged 40 to 49 years.16–17 In general, screening of women aged less than 40 years is restricted to high-risk subgroups (women with a 20% lifetime breast cancer risk at or before the age of 30 years or breast cancer risk at a given age equivalent to that of the average woman at the age of 40 years). These include BRCA1 or BRCA2 gene mutation carriers, women with a personal history of breast cancer, women with a prior diagnosis of atypical ductal or lobular hyperplasia, women with previous radiation therapy to the chest before the age of 30 years, and women with a strong family history of breast cancer (usually involving one or more first-degree relatives with premenopausal breast cancer or breast cancer before the age of 50 years).
LIMITATIONS OF MAMMOGRAPHY
Although there have been significant improvements in mammographic technique over the past 50 years, fundamental limitations remain. These include the low inherent contrast differences between tissue structures in the breast and the fact that mammographic detection of breast cancer (sensitivity) relies on the ability to visualize cancer through the background of overlying normal tissue (Figure 1). Mammographic specificity relies on the ability to distinguish benign from malignant breast lesions based on their margins and morphologic features.18–19 However, malignant and benign lesions may have similar appearances, thereby reducing specificity. Data from the Breast Cancer Surveillance Consortium demonstrated that on average, screening mammography programs had a callback rate of 6.4% to 13.3% and a positive predictive value of 3.4% to 6.2%.20 The mean cancer detection rate was 4.7 per 1000, and the mean size of invasive cancers was 13 mm. The main mammographic signs of breast cancer include clustered microcalcifications, masses, architectural distortions, and asymmetrical densities. Comparison to prior mammograms is essential because some cancers may only be detected by perceiving them as a subtle change from prior studies. In addition, the availability of prior mammograms for comparison can reduce the number of unnecessary callbacks for stable benign findings.21
DIGITAL MAMMOGRAPHY AND COMPUTER-AIDED DETECTION
Screen-film mammography (SFM) has been the standard method used for breast cancer screening since the end of xeromammography some 20 years ago. Advances in screen-film technology and film-processing techniques have contributed to major improvements in the quality of mammographic images. In addition to high contrast, the strength of SFM lies in its extremely high spatial resolution, often greater than 10 line pairs per millimeter (lp/mm). This allows the detection of exceedingly small clusters of microcalcifications, one of the earliest signs of breast cancer. The limitations of SFM include the detection of subtle soft tissue lesions, especially in the presence of dense glandular tissues, and the fact that the film serves simultaneously as the image receptor, display medium, and long-term storage medium. Recent technologic advances have led to the development of full-field digital mammography (FFDM). One of the initial concerns of FFDM is its inherent lower spatial resolution of 5 to 10 lp/mm. However, several studies have demonstrated that despite the limited spatial resolution, the visibility of calcifications on FFDM is not significantly different from that on SFM.22–24 The higher contrast resolution of FFDM may account for its comparable detection rates. In a recent multicenter trial of 49,528 women, Pisano and colleagues24 reported that the overall diagnostic accuracy of FFDM was comparable to SFM as a means of screening for breast cancer. The study also found that digital mammography was more accurate in women younger than 50 years, women with radiographically dense breasts, and premenopausal or perimenopausal women.
CAD programs were developed to assist a radiologist in the interpretation of screening mammograms, so that cancer detection rates could be improved. These programs rely on neural networks to analyze the images and highlight potentially suspicious findings that may have been overlooked by the radiologist. Several studies have shown improved cancer detection by radiologists using CAD versus radiologists alone, without significantly increasing callback rates.24–27 However, other studies have shown less promising results.28–29 A recent study by Fenton and colleagues29 reported that CAD decreased the accuracy of screening mammogram interpretation. This was not due to a decrease in cancer detection, but rather to an increase in false-positive results. To be effective, CAD programs should not increase callback rates and should not significantly prolong interpretation times for screening mammograms. It is important to remember that CAD programs are tools that must be used in an appropriate manner. They should not be used to override a suspicious finding detected by a radiologist. In general, these systems tend to be more helpful for low-volume or inexperienced readers.
MAGNETIC RESONANCE IMAGING
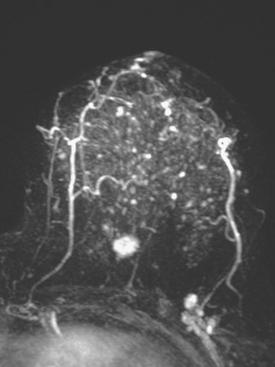
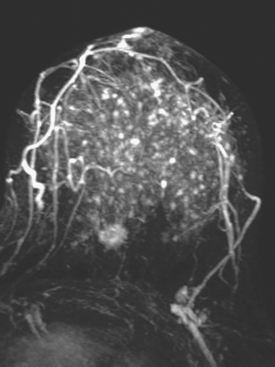
Dynamic, contrast-enhanced MRI of the breast has been shown to be extremely sensitive in the detection of invasive breast cancer and is not limited by the density of the breast tissue. However, because the reported sensitivity of MRI for ductal carcinoma in situ (DCIS) ranges between 45% and 100%, MRI is currently not recommended as a replacement for mammography.30 A more recent single institution study suggests that MRI may have a higher sensitivity than mammography for DCIS than previously thought, particularly for high-grade DCIS.31 The use of MRI in the general population has been limited by its moderate specificity. Therefore, its use has been focused on studying patients in whom the yield from MRI is likely to be higher. Multiple studies have shown that MRI is a useful tool as an adjuvant to screening mammography in women at high risk for breast cancer.32–34 The American Cancer Society (ACS) recommends annual screening MRI for women with a 20% to 25% lifetime risk for breast cancer.35 This includes women with the BRCA1 or BRCA2 breast cancer genes, as well as women with multiple family members with breast or ovarian cancer, and women who have undergone mediastinal irradiation for Hodgkin’s disease. Women whose benefit from screening MRI was considered questionable by the ACS because of insufficient data included women with a personal history of breast cancer, prior biopsy yielding atypia, or extremely dense breasts on mammography. The decision to perform screening MRI in these women should be made on a case-by-case basis. Several models may be used to calculate lifetime risk for breast cancer, including the Gail, Claus, and Tyrer-Cusick models.
ULTRASOUND
Early studies of breast ultrasound for cancer screening were disappointing because of its poor detection of small cancers and excessively high false-positive rates.36–40 With the advent in the early 1990s of technical improvements in ultrasound, including improved spatial and contrast resolution utilizing higher-megahertz (MHz) transducers, the potential of whole-breast ultrasound as a screening tool has been revisited. Several recent singleinstitution studies have demonstrated a prevalence detection rate of 3 to 4 mammographically occult cancers per 1000 women screened.41–50 However, the biopsy positive predictive values were less than 20%, lower than accepted for mammography.51 These initial studies have focused mainly on women with mammographically higher-density breasts. Screening ultrasound appears to be more sensitive in detecting early invasive cancer, whereas mammography is more sensitive in the detection of DCIS. Of the invasive cancers, ultrasound found a higher percentage of invasive lobular carcinomas than that usually found on mammography. Therefore, whole-breast ultrasound should supplement mammographic screening, rather than replace it.
The initial studies were performed mostly by radiologists with a high ultrasound skill level and in some studies were not blinded to the mammographic findings. Therefore, the initial results of screening ultrasound may not extrapolate to its use in general clinical practice.52 Other concerns include the lack of standardized exam techniques, interpretation criteria, false-positive results, and unnecessary biopsies. In addition, most of the initial studies on whole-breast ultrasound have evaluated prevalence (initial) detection rates, rather than incidence (subsequent) detection rates. The benefit from subsequent yearly screening ultrasound is uncertain, but likely to be less. The American College of Radiology Imaging Network (ACRIN) is currently conducting a randomized multicenter trial evaluating whole-breast bilateral screening ultrasound in high-risk, asymptomatic women with dense breasts. The study will evaluate both prevalence (year 1) and incidence (years 2 and 3) screening ultrasound detection rates as compared with mammography. Interpretation of the examinations will be performed by radiologists trained in mammographic and ultrasound interpretation, using standardized interpretive criteria.
1 Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30.
2 Ries LAG, Eisner MP, Kosary CL, et al. SEER cancer statistics review, 1975–2001. Bethesda, MD: National Cancer Institute, 2004.
3 Elkin EB, Hudis C, Begg CB, Schrag D. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975–1999. Cancer. 2005;104(6):1149-1157.
4 Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
5 Smith RA, Duffy SW, Gabe R, et al. The random-ized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793-806. v.
6 Shapiro S, Venet W, Strax P, Venet L. Periodic screening for breast cancer: the Health Insurance Plan Project and its Sequelae, 1963–1986. Baltimore: Johns Hopkins University Press, 1988.
7 Tabar L, Vitak B, Chen HH, et al. The Swedish Two-County Trial twenty years later. Radiol Clin North Am. 2000;38:625-652.
8 Alexander FE, Anderson TJ, Brown HK, et al. 14 years of follow-up from Edinburgh randomized trial of breast cancer screening. Lancet. 1999;353:1903-1908.
9 Andersson I, Aspegren K, Janzon L, et al. Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. BMJ. 1988;297:943-948.
10 Frisell J, Lidbrink E, Hellstrom L, Rutqvist LE. Follow-up after 11 years: update of mortality results in the Stockholm mammographic screening trial. Breast Cancer Res Treat. 1997;45:263-270.
11 Bjurstam N, Bjorneld L, Duffy SW. The Gothenburg Breast Screening Trial: first results on mortality, incidence, and mode of detection for women ages 39–49 years at randomization. Cancer. 1997;80:2091-2099.
12 Hendrick RE, Smith RA, Rutledge JH3rd, Smart CR. Benefit of screening mammography in women aged 40–49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
13 Chu KC, Smart CR, Tarone RE. Analysis of breast cancer mortality and stage distribution by age for the Health Insurance Plan clinical trial. J Natl Cancer Inst. 1988;80:1125-1132.
14 Bjurstam N, Bjorneld L, Warwick J, et al. The Gothenburg Breast Screening Trial. Cancer. 2003;97:2387-2396.
15 Andersson I, Janzon L. Reduced breast cancer mortality in women under 50: updated results from the Malmo Mammographic Screening Program. J Natl Cancer Inst Monogr. 1997;22:63-68.
16 Liberman L, Dershaw DD, Deutch BM, et al. Screening mammography: value in women 35–39 years old. AJR Am J Roentgenol. 1993;161:53-56.
17 Curpen BN, Sickles EA, Sollitto RA, et al. The comparative value of mammographic screening for women 40–49 years old versus women 50–64 years old. AJR Am J Roentgenol. 1995;164:1099-1103.
18 Sickles EA. Breast masses: mammographic evaluation. Radiology. 1989;173(2):297-303. Review
19 Sickles EA. Mammographic features of malignancy found during screening. Recent Results Cancer Res. 1990;119:88-93. Review
20 Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology. 2006;241:55-66.
21 Frankel SD, Sickles EA, Curpen BN, et al. Initial versus subsequent screening mammography: comparison of findings and their prognostic significance. AJR Am J Roentgenol. 1995;164:1107-1109.
22 Fischer U, Baum F, Obenauer S, et al. Comparative study in patients with microcalcifications: full-field digital mammography vs screen-film mammography. Eur Radiol. 2002;12:2679-2683.
23 Lewin JM, Hendrick RE, D’Orsi CJ, et al. Comparison of full-field digital mammography to screen-film mammography for cancer detection: results of 4945 paired examinations. Radiology. 2001;218:873-880.
24 Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773-1783.
25 Freer TW, Ulissey MJ. Screening mammography with computer-aided detection: prospective study of 12,860 patients in a community breast center. Radiology. 2001;220(3):781-786.
26 Birdwell RL, Bandodkar P, Ikeda DM. Computer-aided detection with screening mammography in a university hospital setting. Radiology. 2005;236(2):451-457.
27 Morton MJ, Whaley DH, Brandt KR, Amrami KK. Screening mammograms: interpretation with computer-aided detection—prospective evaluation. Radiology. 2006;239(2):375-383.
28 Gur D, Sumkin JH, Rockette HE, et al. Changes in breast cancer detection and mammography recall rates after the introduction of a computer-aided detection system. J Natl Cancer Inst. 2004;96(3):185-190.
29 Fenton JJ, Taplin SH, Carney PA, et al. Influence of computer-aided detection on performance of screening mammography. N Engl J Med. 2007;356(14):1399-1409.
30 Bazzocchi M, Zuiani C, Panizza P, et al. Contrast-enhanced breast MRI in patients with suspicious microcalcifications on mammography: results of a multicenter trial. AJR Am J Roentgenol. 2006;186:1723-1732.
31 Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370(9586):485-492.
32 Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427-437.
33 Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103:1898-1905.
34 Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469-8476.
35 Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89.
36 Cole-Beuglet C, Goldberg BB, Kurtz AB, et al. Clinical experience with a prototype realtime dedicated breast scanner. AJR Am J Roentgenol. 1982;139:905-911.
37 Sickles EA, Filly RA, Callen PW. Breast cancer detection with sonography and mammography: comparison using state-of-the-art equipment. AJR Am J Roentgenol. 1983;140:843-845.
38 Egan RL, Egan KL. Automated water-path full-breast sonography: correlation with histology of 176 solid lesions. AJR Am J Roentgenol. 1984;143:499-507.
39 Egan RL, McSweeney MB, Murphy FB. Breast sonography and the detection of breast cancer. Recent Results Cancer Res. 1984;90:90-100.
40 Kopans DB, Meyer JE, Lindfors KK. Whole-breast US imaging: four-year follow-up. Radiology. 1985;157:505-507.
41 Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound. Cancer. 1995;76:626-630.
42 Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening US—diagnostic yield and tumor characteristics. Radiology. 1998;207:191-199.
43 Buchberger W, DeKoekkoek-Doll P, Springer P, et al. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR Am J Roentgenol. 1999;173:921-927.
44 Buchberger W, Niehoff A, Obrist A, et al. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR. 2000;21:325-336.
45 Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001;221:641-649.
46 Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175.
47 Leconte I, Feger C, Galant C, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol. 2003;180:1675-1679.
48 Crystal P, Strano S, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003;181:177-182.
49 Cortesi L, Turchetti D, Marchi I, et al. Breast cancer screening in women at increased risk according to different family histories: an update of the Modena Study Group experience. BMC Cancer. 2006;6:210.
50 Corsetti V, Ferrari A, Ghirardi M, et al. Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol Med (Torino). 2006;111(3):440-448.
51 Quality Determinants of Mammography Guideline Panel. Quality determinants of mammography. AHCPR Publication no. 95–0632. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, 1994.
52 Berg WA, Blume JD, Cormack JB, Mendelson EB. Operator dependence of physician-performed whole-breast US: lesion detection and characterization. Radiology. 2006;241(2):355-365.
CASE 1 Breast cancer presenting as a small new mass on mammography
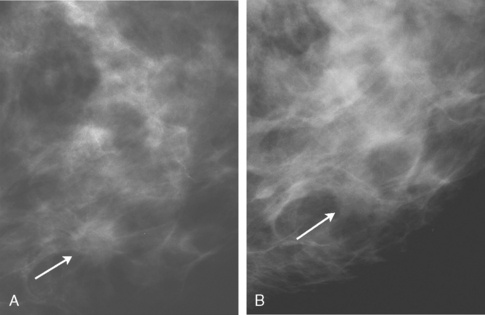
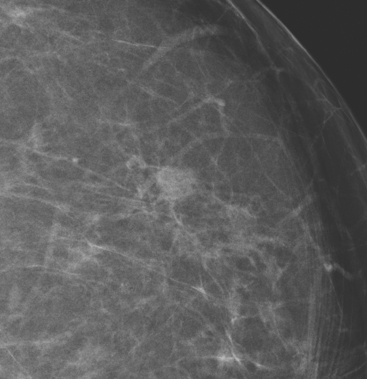
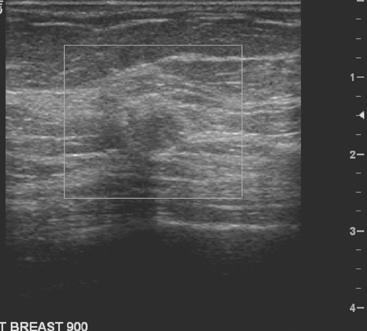
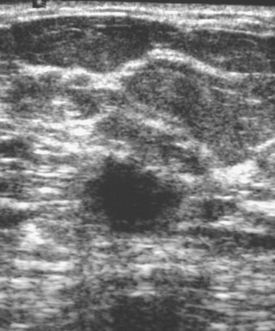
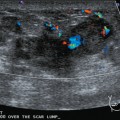
An asymptomatic 57-year-old woman underwent screening mammography, which showed a new finding of a 6-mm round mass in the anterior upper outer quadrant (UOQ) of the left breast (Figures 1 and 2). The patient had undergone a prior benign left breast needle biopsy with placement of a marker clip in the posterior upper outer left breast. Ultrasound confirmed the presence of a somewhat ill-defined 6-mm solid mass (Figure 3). Ultrasound-guided core needle biopsy yielded a diagnosis of low-grade ductal carcinoma in situ.
CASE 2 Breast cancer presenting as a new mass on mammography
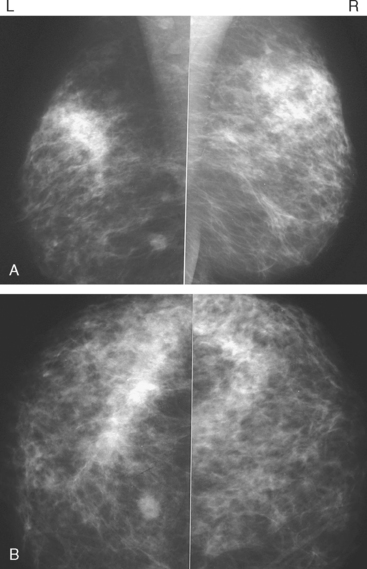
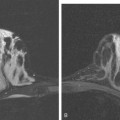
A new left lower inner quadrant mass with ill-defined margins was identified on a screening mammogram performed on an asymptomatic 67-year-old woman (Figure 1). Sonography confirmed a suspicious, 1 cm, irregularly marginated, hypoechoic, solid mass, which was taller than wide (Figure 2). A mammographically questioned second abnormality, architectural distortion in the left upper breast, had no sonographic confirmation. MRI was recommended to further assess the question of possible multicentric disease. The suspected cancer mass was highly suspicious by MRI criteria, showing irregular margination (Figures 3 and 4) and a washout pattern of enhancement (Figure 5). No correlate was found on MRI for the questioned left upper architectural distortion. There was no evidence of multicentricity by MRI.