The cerebral cortex develops in several stages from a pseudostratified epithelium at 5 weeks to an essentially complete cortex at 47 weeks. Cortical connectivity starts with thalamocortical connections in the 3rd trimester only and continues until well after birth. Vascularity adapts to proliferation and connectivity. Malformations of cortical development are classified into disorders of specification, proliferation/apoptosis, migration, and organization. However, all processes are intermingled, as for example a dysplastic cell may migrate incompletely and not connect appropriately. However, this classification is convenient for didactic purposes as long as the complex interactions between the different processes are kept in mind.
The development of the cerebral cortex results from complex and overlapping processes of cellular proliferation, differentiation, and apoptosis, of migration, and of organization (development of neuronal connections). The term malformation of cortical development (MCD) describes the structural abnormality resulting from any defect affecting any stage of this development. From a terminological point of view, it is now preferred to the name “migration defect,” which is too specific (the cortex may be malformed even when the cells have migrated normally). The term cortical dysgenesis would be appropriate as well. The term “cortical dysplasia” would be acceptable, but it has been in use since as early as 1971 to describe a specific variety of cortical malformation.
MCD have been known for a long time (see Refs. for review), but it is only after the introduction of modern imaging modalities (computed tomography [CT], but above all magnetic resonance [MR] imaging) that their clinical importance has been recognized as a major cause of developmental delay, refractory epilepsy, and cerebral palsy. When the development of the brain was not yet clearly understood, they were described by pathologists according to the morphologic feature that was the most striking when looking at the brain, such as the size or the appearance of the brain surface (eg, small or big: microencephaly or megalencephaly; smooth: lissencephaly-agyria-pachygyria, cobblestone brain; too many small gyri: polymicrogyria; cavities: porencephaly-schizencephaly). This terminology has remained despite a much better understanding of the pathogenetic processes. As soon as the present model of development was established, with its phases of proliferation-apoptosis, migration, and organization, the malformations were classified accordingly, while the names were retained : microencephaly and megalencephaly as proliferation disorders; heterotopia and later lissencephaly as migration disorders; polymicrogyria and schizencephaly (which includes polymicrogyric cortex) as organization disorders. It must be mentioned that such a classification introduces a bias in the understanding of the MCD, as it suggests that the malformations would develop sequentially: proliferation disorders early, migration disorders later, and organization disorders last, which would be an oversimplification.
In the last two decades the progressive unraveling of the role of specific genetic cascades that control the development of the cortex, and the identification in several entities of corresponding genetic defects, have opened new avenues to understanding the MCD. Putting all those facts together, Barkovich and colleagues have proposed a morphologic and tentatively pathogenetic classification of MCD, which includes the developmental glioneuronal tumors in MCD (like the classification of Raymond and colleagues before) and subdivides focal cortical dysplasia (FCD) into two clearly separate groups depending on whether they present with dysmorphic/balloon cells (considered as malformed cells related to an abnormal neuronal and glial genesis), or with architectural changes only (considered the result of an abnormal cortical organization). This classification, updated in 2001 and 2005, is the most widely accepted today ( Box 1 ).
- 1.
Malformations due to abnormal neuronal and glial proliferation or apoptosis
- a.
Abnormality of brain size: decreased proliferation/increased apoptosis or increased proliferation/decreased apoptosis:
- i.
Microcephaly with normal to thin cortex
- ii.
Microlissencephaly (extreme microencephaly with thick cortex)
- iii.
Microcephaly with extensive polymicrogyria
- iv.
Macrocephalies
- i.
- b.
Abnormal proliferation
- i.
Nonneoplastic
- 1.
Cortical tubers of tuberous sclerosis
- 2.
Focal cortical dysplasia with balloon cells
- 3.
Hemimegalencephaly
- 1.
- ii.
Neoplastic (associated with disordered cortex)
- 1.
Dysembryoplastic neuroectodermal tumor (DNET)
- 2.
Ganglioglioma
- 3.
Gangliocytoma
- 1.
- i.
- a.
- 2.
Malformations due to abnormal neuronal migration
- a.
Lissencephaly/band heterotopia spectrum
- b.
Cobblestone cortex complex
- c.
Heterotopia
- i.
Periventricular nodular heterotopia
- ii.
Subcortical nodular heterotopia
- iii.
Marginal glioneuronal heterotopia
- i.
- a.
- 3.
Malformation due to abnormal cortical organization/late neuronal migration
- a.
Polymicrogyria and schizencephaly
- i.
Bilateral polymicrogyria syndromes
- ii.
Schizencephaly (polymicrogyria with clefts)
- iii.
Polymicrogyria or schizencephaly as part of multiple congenital anomaly/mental retardation syndromes
- i.
- b.
Focal cortical dysplasia without balloon cells
- c.
Microdysgenesis
- a.
- 4.
Malformations of cortical development not otherwise classified
- a.
Malformations secondary to inborn errors of metabolism
- i.
Mitochondrial and pyruvate metabolic disorders
- ii.
Peroxisomal disorders
- i.
- b.
Other unclassified malformations
- i.
Sublobar dysplasia
- ii.
Others
- i.
- a.
Although this article fully adheres to this classification, the authors mildly diverge from it in dealing with FCD: it is described first and separately as a single group because (whatever their pathogenesis) all present in a similar and specific clinical, radiological, and surgical context. The authors then resume the classical approach and proceed with the groups of disorders of proliferation/apoptosis, of migration disorders (including the lissencephalies), and of schizencephaly and polymicrogyria. Before that, an introductory review of the imaging approach and of the development is provided.
The imaging approach
What to Look for
MCD are primarily disorders of the cerebral cortex, and imaging therefore should carefully investigate the cortex: sulcal/gyral pattern (all primary and secondary sulci bear names and can be identified; they are essentially symmetric) and depth (symmetric); cortical thickness (normally 1 mm in the depth of the sulcus, 2–3 mm at the crown of the gyrus) and demarcation from the white matter; T1 and T2/fluid-attenuated inversion recovery (FLAIR) signals. In addition, the white matter originates from and is functionally associated with the cortex, and it is traversed by the migration path of the cortical cells: it may have become dysplastic together with the cortex (abnormal cellularity, heterotopic neurons or monstrous cells, abnormal connectivity) as a part of the cortical malformation, or its development may have been secondarily altered by the cortical abnormality (distorted plasticity); it may also have become abnormal as a late result of the seizure activity of the overlying cortex (demyelination, gliosis). As the connectivity often is decreased, so is the volume of the white matter: this may be expressed by the volume of the brain, of one hemisphere or lobe, by the size and morphology of the ventricle(s), or by the asymmetry of the brainstem. Finally, many of the genes that control the cellular migration to and fate in the cerebral cortex may also control the development of the basal ganglia, thalami, and brainstem nuclei, of the cerebellar cortex, and of the cord. Some even may be involved in the development of extraneural tissues (eg, cobblestone cortex and deficient white matter associated with congenital muscular dystrophy).
The Tools
Conventional imaging
The clinical importance of the MCD appeared as soon as CT became the primary diagnostic modality in Neuroradiology (mid-1970s), but in this field like elsewhere, the imaging modality of choice is MR. The “best protocols” are many, depending on the type of equipment available, on the technological advances, and on the familiarity of the neuroradiologist with the pathology. Whatever is preferred, the study should always be multisequential and multiplanar, using high spatial definition and high contrast resolution (high signal-to-noise [S/N]). Because the abnormalities often are subtle, the most recent and most efficient machine should be used, and older cases of “nonlesional” epilepsies should be reinvestigated when the equipment is changed or updated. Obviously 3-T magnets have a better resolution power than 1.5-T magnets; multiple-phased array coils with parallel imaging are better than conventional coils, providing high S/N images while keeping the acquisition time within reasonable limits. Most modern neuroimaging centers are now equipped with PACS (picture archiving and communications system); if not, digital storage of the image is mandatory, rather than conventional films, because small, subtle lesions may escape the first review of the images and may be identified after repeated and careful review only. As much as possible, the examination should be directed by the clinical and electrographic/magnetoencephalographic (MEG) data, and if available, the results of the functional studies (single-photon emission CT [SPECT], positron emission tomography [PET]): an oriented study is more likely to be productive than a blind study.
The basic conventional sequences are T1, T2, and FLAIR. T1 images ideally should be from volumic acquisition (MP-RAGE/TFE/SPGR), with 1- to 1.5-mm partitions to minimize the partial volume effects. The thickness and demarcation of the cortical ribbon are better appreciated, and focal cortical signal changes can be securely identified. The typical acquisition plane is the sagittal plane, with reformatting in whatever other plane is felt necessary, even curvilinear planes. The images provide an excellent spatial resolution and an excellent gray-white contrast, allowing for a superb anatomic study. To analyze the suprasylvian sulcal pattern, the sagittal and axial planes are the best; for the temporal structures, especially the mesial ones, coronal slices perpendicular to the long axis of the hippocampus are optimal. The cortical-subcortical definition, however, is poor in infants between the ages of a few weeks and at least 1 year.
T2 imaging is still important for anatomy, and to evaluate the microstructural changes in the parenchyma. The plane(s) should be adapted to the location of the expected abnormality; in a routine protocol, coronal and axial planes are usually chosen. Some groups prefer using true T2 spin echo (T2SE), whereas most use conventional T2 fast-spin echo (T2FSE). Some also advocate the use of 3-dimensional (3D)-FSE with 1-mm partition, and slices certainly should not be thicker than 2 to 3 mm, depending on the equipment available. In the neonatal and in the mature brain, T2-weighted imaging (T2WI) is excellent at showing the cortical involvement, the cortical thickness, and the cortical-subcortical blurring, if any.
FLAIR imaging certainly is the sequence most sensitive to structural changes, but its spatial resolution is not as good as that of T2 turbo-spin echo (T2TSE), with artifacts from flowing blood or cerebrospinal fluid (CSF) being more common. Yet it is practically the first sequence to be looked at, as it will readily demonstrate any significant abnormality of signal. Like conventional T2WI, 2- to 3-mm slices should obtained, typically axial and coronal, or even 1-mm slices using 3D FLAIR. It demonstrates changes in both the cortex and the white matter, and a cortical blurring as well. It is of limited use, however, in young children. Some groups like to use proton density images instead, or in complement of FLAIR.
Besides the main conventional sequences, other sequences may be useful in specific instances. Susceptibility sequences (SWI) are useful to demonstrate that an epileptogenic dysplasia is associated with vascular abnormalities such as a cavernoma or a meningoangiomatosis. Diffusion imaging (DWI/ADC) is not very contributive in the assessment of MCD: it is typically not sensitive enough to show the microstructural changes of the tissue, which will be much more confidently evaluated by quantitative diffusion tensor imaging (DTI); however, it may demonstrate restriction due to cytotoxic edema in case of refractory seizure activity. MR venography may be used to illustrate venous abnormalities commonly associated with some MCD such as a polymicrogyria (PMG), whereas MR arteriography may show abnormal arterial patterns.
Imaging in infants
In infants and young children (as well as in developmentally delayed children) good MR imaging implies the use of sedation/general anesthesia. During the first months of life the structure of the immature brain tissue is different and the T1 relaxation time is much longer than in the mature brain, so that adapted sequences should be used. T1-weighted (T1W) sequences need a longer repetition time (TR), and T2W sequences need longer TR and echo time. Also, as mentioned earlier, if the cortex is exquisitely delineated at birth on T1 and T2, the contrast becomes lost with advancing myelination, until after 1 year on T1, and 2 years on T2. On FLAIR images the evolution is still more complex: at birth, the very high water content of the white matter is cancelled by the saturation pulse and the appearance is somewhat similar to T1; in the following weeks the myelin precursors accumulate and the signal increases to look more like a conventional T2. However, it remains fairly heterogeneous for a much longer time than on ordinary T2 sequences, as the mature pattern is not reached until about the age of 3 to 4 years. In summary, the optimal time to identify a cortical dysplasia in an infant is either early in the first weeks or much later when the mature pattern is established after 2 years, and FLAIR imaging is of limited use in young children.
Another specificity of epileptic infants is the possible occurrence of a focally accelerated myelination. Many investigators have observed that when FCD is diagnosed in the first months of life the white matter under the dysplastic cortex displays a low T2 signal, which eventually disappears while maturation proceeds. Most assumed that this appearance was a feature inherent to FCD, a reflection of an associated white matter dysplasia, and/or possibly microscopic calcification, but it was also suggested that it could result from the seizure activity itself. This idea is supported by the experimental evidence that electrical activity in the axons induces the myelination, and that increasing this activity increases the myelination, a process mediated by astrocytes. Accelerated myelination is observed in other epileptogenic conditions such as the Stürge-Weber syndrome. Finally, it explains why the change is no longer apparent when myelination is completed: this would not be expected to happen in the case of white matter dysplasia. It is important to keep in mind that as a consequence, the early myelination points to an epileptogenic focus and not necessarily to an FCD.
Special MR imaging techniques
Because of the high percentage of lesions that are not well demonstrated on MR imaging, different approaches have been proposed to increase the diagnostic yield. Various MR techniques are available to provide more insight into the structure, function, and metabolism of the epileptic brain affected with an MCD. Quantitative MR demonstrates that T2 correlates with the neuronal density in the cortex. Volumetric studies suggest a relative defect of the white matter, hence of the connectivity. DTI is being used extensively. Quantitative DTI is more sensitive than DWI/ADC to demonstrate a decreased diffusivity postictally, matching the epileptic focus, apparently related to a cellular swelling due to the metabolic exhaustion. It also identifies an increased diffusivity interictally, which may be due to neuronal loss and gliosis in both gray and white matter, as well as to a dysgenetic structural alteration. DTI tractography demonstrates more extensive changes in the brain organization and connectivity beyond the cortical lesion. Proton spectroscopy ( 1 H-MRS) is not very useful for the diagnosis of an MCD composed of normal if ill-located or ill-organized neurons and glia, but it provides insights on their metabolism. N -Acetylaspartic acid (NAA) appears largely unchanged or only mildly decreased in heterotopia and PMG, choline appears either increased or normal, and glutamate and γ-aminobutyric acid (GABA) appear increased in patients with epileptogenic heterotopias and PMG. In lesions made of poorly differentiated cells such as in FCD or in the tubers of tuberous sclerosis complex (TSC), NAA appears significantly decreased and choline increased, but less so than in cerebral tumors. Perfusion also is low in cortical tubers but normal in PMG. Despite it having been proved useful in the evaluation of the cortical and white matter dysplasia in tuberous sclerosis, there is no report on the potential use of magnetization transfer imaging (MTI) in MCD in general, and in FCD in particular. One report, however, indicates that in acquired and developmental epileptogenic lesions, significantly reduced magnetization transfer ratio (MTR) was found within the MR-visible lesions (presumably gliosis with low myelin content) as well as in normal-looking white matter. These areas concurred with the electrographic epileptic activity and the clinical seizure semiology.
Cortical Function and White Matter Organization In and Around MCD: Presurgical Assessment
Imaging has become essential in preparing for epilepsy surgery, if warranted. It is expected to show the lesion, identify its nature and, as much as possible, its extent. Quantitative DTI shows microstructural changes beyond the abnormalities seen on conventional MR images, in good correlation with the MEG abnormalities.
Imaging is also expected to tell whether the lesion is functional or not. MCD have an intrinsic epileptogenicity, which implies that they are interconnected with the rest of the brain; some patients with MCD present with reflex epilepsy, which means that the lesion can be activated by outside stimuli (for review see Ref. ). Using functional imaging (fMRI), one study demonstrated that 64% of MCD are activated by simple sensory motor or visual stimuli (71% if they are located in the corresponding eloquent areas), but only 40% become involved in complex cognitive tasks. However, the response of the dysgenetic cortex depends on the severity of the malformation: all cases of organization disorders (PMG, schizencephaly, and FCD type I) become activated by simple tasks against only 47% of cases of FCD type II (Taylor) and heterotopias; this is in good agreement with the current classification of MCD (see Box 1 ).
MR imaging techniques are also used to locate the main cognitive functions and white matter tracts. fMRI has, in practice, completely supplanted the sodium amobarbital Wada test. It is used to locate the motor function when surgery in the sensory-motor area is considered, and to locate the language representation when the lesion to be operated on is in the so-called dominant hemisphere, as language representation is commonly atypical not only in MCD but more generally in refractory epilepsy. Assessment and lateralization of memory functions can be done, but need validation and are not performed routinely as yet. Finally, DTI tractography is an efficient way of locating the major axonal tracts such as the corticospinal tract or the optic radiations. Some groups are attempting to demonstrate the language networks and the memory networks as well.
As mentioned earlier, a focused MR study is more efficient than a blind one, and a multimodality approach enhances the diagnostic efficacy in demonstrating the lesional as well as the epileptogenic area: clinical semiology, scalp electroencephalography (EEG), coregistered MEG and magnetic source imaging (MSI), or functional neuroimaging such as postictal/interictal SPECT and PET are extremely productive. A new approach using EEG-correlated fMRI is being developed and seems promising.
Development of the cerebral cortex
Cortical Anatomy
The cerebral cortex comprises the trilayered olfactory paleocortex and hippocampal archicortex, and an extensive 6-layered neocortex (90% of the cortical surface in human). In advanced mammals and especially in humans, it is conspicuously folded, two-thirds of the cortical surface being located inside the sulci; cortical folding is related to the development of the connectivity. The sulci have been classified into primary sulci (pericallosal, cingulate, parieto-occipital, hippocampal sulci) and secondary sulci (such as the central, precentral and postcentral, intraparietal, frontal, temporal, calcarine and occipital sulci). The primary and secondary sulci may vary in shape slightly but are constant and symmetric in location. The sulci delineate the gyri, which more or less reflect the functional areas of Brodmann. The primary sulci become apparent shortly after mid-gestation, and the secondary sulci appear between 25 and 30 weeks. Tertiary sulci are branches of the primary and secondary sulci and appear mostly after birth; they are extremely variable.
The thickness of the neocortex varies from 1 to 3 mm, thinner in the depth of the sulci and thicker at the crown of the gyri. Pyramidal neurons (glutamatergic, excitatory) are the most numerous (80%) and establish long-range connections; interneurons (GABAergic, inhibitory) establish local, intracortical connections between the pyramidal neurons. The neurons are primarily organized in columnar units, but because of the intracortical course of the connecting fibers they become organized in layers. From the surface to the depth, the neocortical layers are as follows, albeit with some overlapping between them:
- •
Layer 1 or molecular layer contains mostly local connecting fibers
- •
Layer 2 receives corticocortical afferents (association and commissural fibers)
- •
Layer 3 sends corticocortical efferents (association and commissural fibers)
- •
Layer 4 or granular layer receives the corticothalamic afferents
- •
Layer 5 or pyramidal layer sends the cortico-subcortical efferents (to the striatum, brainstem, and cord)
- •
Layer 6 or polymorphic layer sends the corticothalamic efferents.
The 6-tier layering of the cortex is due to the predominantly horizontal organization of the intracortical fiber tracts. The most prominent fiber layers are in layer 1, in layer 4 (external band of Baillarger), and between layers 5 and 6 (internal band of Baillarger). If the general cortical pattern is constant, the proportion between the layers varies according to the cortical location, resulting in the various histologic patterns that characterize the cortical functional areas of Brodmann.
Formation of the Cortex
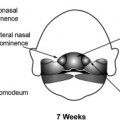
In the last 4 decades a considerable amount of information has accumulated regarding the development of the cortex, notably in the last decade (for reviews see Refs. ) ( Fig. 1 ). The early central nervous system emerges from the surface ectoderm as a band of dorsal midline neuroepithelium or neural plate during the third week (for review see Ref. ). This neural plate forms a groove with neural folds and closes to form the neural tube (NT) during the fourth week (neurulation); 3 cerebral vesicles (forebrain, midbrain, and hindbrain) become apparent during the fifth week, and the lateral evaginations of the cerebral hemispheres develop from the forebrain during the sixth week. Under the influence of ventralization and dorsalization factors, the hemispheric vesicles become divided into a basal part or subpallium (future basal ganglia) and a dorsal part or pallium (future cortex and white matter), each with its own germinal zone, the dorsal one producing pyramidal neurons and the basal one (ganglionic eminence) producing the cortical interneurons as well as the neurons of the basal ganglia (in humans interneurons may come from the pallial germinal zone as well). The division is clearly apparent during the seventh week.
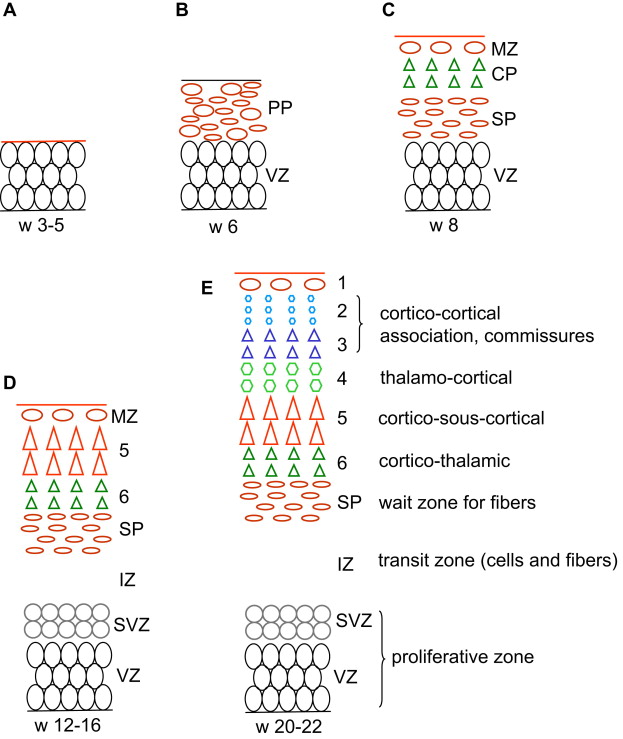
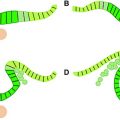
Proliferation of neuroepithelial, truly neural stem cells already begins in the fourth week in the neural plate. As the NT closes, its whole thickness forms a proliferating zone where the cells divide in a symmetric way (one stem cell produces two stem cells) (see Fig. 1 A). At the end of the fifth week the proliferation process switches to asymmetric divisions (one stem cell produces one stem cell and one neuron) and the differentiated neurons accumulate at the periphery: as a consequence the wall of the NT contains a deep germinative zone, which is called the ventricular zone (VZ), and a peripheral zone with the first neurons, which is called the primordial plexiform layer or preplate (PP) (see Fig. 1 B). The distance between the ventricular and the meningeal surfaces of the NT is short in the early stages, and the differentiated cells are able to migrate by somal translocation (nucleokinesis): from the germinal zone where they are born they extend a process toward the meningeal surface, the nucleus migrates into this process toward the surface while the ventricular process shortens and loses its ventricular contact. One of the genes that controls this nucleokinesis is the LIS1 gene, whose defect is associated with one major form of lissencephaly. The process of translocation is used by the neurons of the PP and possibly by the early neurons (future layer 6) of the cortical plate (CP). The PP contains Cajal-Retzius cells and other neurons that are the first to establish extracortical connections. When the cortical plate appears on about day 50 (end of week 7), it divides the PP into two layers: the superficial layer or marginal zone (MZ) contains the reelin-positive Cajal-Retzius cells (in addition to various other neurons), and the subcortical layer forms the subplate (SP) and contains reelin-negative neurons (see Fig. 1 C). Cajal-Retzius cells play a major role of controlling the migration of the neurons in the CP; the subplate is essential also, as it directs outgoing axons and maintains transient connections with the incoming axons until the cortex becomes ready.
Radial migration of glutamatergic pyramidal neuron to the CP begins at the end of week 7 in the lateral part of the telencephalon, and a week later in its posteromedial aspect; the peak migratory activity lasts until mid-gestation (weeks 20–22) and migration is essentially complete before the third trimester. Radial migration uses specialized cells, the radial glia, to guide the pyramidal neuron from the germinal zone to the CP. Each radial glial cell has a process anchored on the ventricular surface, and a radial process that extends to the pial basement membrane (where it often makes contact with vessels ), so that the radial glia forms a scaffolding across the mantle. The newly generated neurons travel perpendicular to the surface, from the pallial VZ along the glial fibers to the CP, where they are induced to detach from the radial glia by the signal (Reelin) provided by the Cajal-Retzius cells. As a consequence early-migrating cells are in the deep cortical layers and late-arriving cells are close to the surface: this is called the inside-out pattern (see Fig. 1 C–E). A first wave of migration toward the deep layer 6 develops at about 7 to 11 weeks; a second wave toward layer 5 occurs at about 12 to 16 weeks; a third and last wave to the more superficial layers occurs after 16 weeks. This last wave is prominent in primates, including man, and corresponds to the neurons that will develop corticocortical connections. Late migration of individual neurons may continue even after the end of the cellular proliferation until after birth. As a consequence of the radial migration, all neurons using a single radial cell form a single column in the CP. The glia-guided migration depends on the cellular microfilament network, and involves the FLN1 gene (whose defect has been demonstrated in specific varieties of gray matter heterotopia), as well as on two proteins associated with the regulation of the actin network, Cdk5 and p35. Doublecortin (DCX) also is involved with radial radiation, and defects of the DCX gene are associated with some human lissencephalies (for review see Ref. ).
Besides their role of guidance, the radial glia recently have been shown to be stem cells and to produce neuronal progenitors. In a rather complex process, they divide asymmetrically in the VZ, producing another radial glial cell and a neuronal progenitor. The neuronal progenitor moves to the subventricular zone (SVZ) (phase 1) where it stays for up to 24 hours, becomes multipolar, and establishes multiple cellular contacts, moves tangentially free from radial glial attachment, and becomes dispersed within the SVZ before dividing symmetrically (phase 2). The new neurons may migrate directly to the cortex along the radial glia, but most translocate back to the VZ (phase 3), from where they make their final journey toward the CP along the radial glial fibers (phase 4).
Tangential migration of the GABAergic inhibitory interneurons occurs in close association with the radial migration of the pyramidal neurons (the first interneurons are seen at about weeks 6–7, even before the appearance of the CP ). These neurons typically originate in the ventral VZ of the medial ganglionic eminence (MGE; the primordium of the globus pallidus ) and travel parallel to the surface of the hemisphere toward the pallium. Like the radial migration, the tangential migration is complex (for review see Ref. ). Studies in rodents show that there are primarily two migration streams, one along the MZ and the other along the deep IZ/SVZ (the intermediate zone [IZ] is the portion of the pallium that is located between the SP and the germinal SVZ). From their MGE origin, some interneurons disperse into the IZ/SVZ before reaching the CP either radially or obliquely; some travel along the MZ and enter the CP from above; some travel in the IZ/SVZ, then reach the MZ radially across the CP, disperse into the MZ, and enter the CP from above. However, the majority of interneurons (70%) travel through the IZ/SVZ and dive toward the ventricle to enter the VZ, where they pause before resuming their course and migrating radially to the CP. In this process, the interneurons are likely to acquire laminar address information, possibly mediated by GABA information. The partial convergence during their migration of at least some pyramidal neurons and interneurons might allow transmission of positional information. Pyramidal neurons pause in the SVZ, become multipolar, and may contact interneurons there. The different migration speed—10 μm/h for the radial migration, 50 μm/h for the tangential ones—may favor birth-date related encounters, and cellular birth date relates to laminar position precisely. What guides the interneurons—guiding glia, axons projecting from the CP—is not clear, but the process has been shown to involve class 3 semaphorins, neuropilins, cell-adhesion molecules, neuroregulins, and the slit/robo complex.
The organization of the pallium changes and becomes more complex as it develops ( Table 1 ), and so does the terminology (see Fig. 1 ). In weeks 4 to 5 the pallium is a simple homogeneous pseudostratified neuroepithelium. Between week 5 and week 7 (before the appearance of CP) it comprises a deep germinal zone VZ and a superficial postmitotic zone PP. After the CP divides the PP during week 8, the postmitotic zone is made up of 3 layers (MZ, CP, and SP), the germinal zone is made up of 2 layers (VZ and SVZ), and an IZ in between contains migrating cells, radial glia processes, and early incoming and outgoing axons. After peaking before mid-gestation, migration stops at about 25 to 27 weeks. The radial glia loses contact with the ventricle, migrates toward the cortex, and forms astrocytes (changing its nestin and PAX6 expression for glial fibrillary acidic protein). The pallial VZ disappears leaving the unicellular layer of ependyma only, but the SVZ persists and contains stem cells even in the adult brain, presumably a potential source of brain tumor cells. The germinal zone of the lateral ganglionic eminence remains prominent for some time (the so-called germinal matrix of the premature brain), before vanishing progressively during the last prenatal weeks. After a peak of complexity between 18 and 28 weeks, the cellularity of the MZ, notably the Cajal-Retzius cells, regresses and disappears before term. On the contrary, the SP expands significantly until the third trimester, being largest at week 28, particularly under the frontal associative cortex, and then attenuates until about term, leaving interstitial neurons only in the white matter. As connectivity develops, white matter fibers progressively invade both the IZ and the SP area, which together form the final hemispheric white matter.
| Age in Weeks | Forebrain | Cellular Processes | Organization of Pallium | White Matter | Metabolic Supply/Vasculature |
|---|---|---|---|---|---|
| 3 | Neural plate | Stem cell proliferation | Pseudostratified | — | Amniotic fluid |
| 4 | Anterior neural plate neural tube closure | Stem cell proliferation | Pseudostratified | — | Primitive meninges |
| 5–6 | Prosencephalon then hemispheres (pallium/subpallium) | First peripheral primordial neurons | VZ and PP | — | Choroid plexus |
| 7–9 | Pallium | Translocation First wave to layer 6 | Postmitotic: MZ, CP, SP Germinal: VZ | Corticothalamic Corticospinal | Choroid plexuses First perforators to VZ/MGE |
| 10–12 | Pallium | Radial glia Progenitors Second wave to layers 5–4 | Postmitotic: MZ, CP, SP Intermediate: IZ Germinal: VZ–SVZ | First thalamocortical in SP | Choroid plexuses Rich germinal plexus SVZ/VZ/MGE |
| 16–20 | Pallium | Third wave to layers 3–2 | — | Thalamocortical in SP Commissural and association in SP | Rich germinal plexus SVZ/VZ/MGE |
| 22–26 | Pallium Primary and early secondary sulci | End of migration and of radial glia | Disappearance of VZ Ependyma | Thalamocortical in layer 4 Early commissural and association to layers 2–3 | Rich germinal plexus SVZ/MGE Early branches in deep cortex |
| 27–32 | Pallium Secondary sulci | — | Reaches mature appearance Prominent SP | Layer 1 and Baillarger Commissural and association to layers 2–3 | Germinal plexus recedes in SVZ Growing vasculature in deep cortex |
| 33–40 | Pallium Developing tertiary sulci | — | SP recedes White matter in IZ/SP | Short association to layers 2–3 | Radial vasculature in superficial cortex End of germinal plexus SVZ/MGE |
| 42–47 | Pallium Developing tertiary sulci | — | End of frontal SP | Short association to layers 2–3 | Steep increase of CBF |
Cellular apoptosis cannot be dissociated from proliferation and organization. The number of neurons in the brain peaks at week 28, but as many as 50% die through apoptosis before the end of the adolescence. Two main periods of apoptosis occur prenatally. The first lasts from week 7 to week 13, and involves proliferating progenitors and young neurons in the VZ. The second, regulated by synaptic activity, cellular contacts, and glial and neuronal trophic factors, eliminates neurons within the CP itself between week 19 and week 23.
Cortical Organization and Developing Connectivity
The period after 22 weeks is the period of organization and differentiation of the cortex ( Fig. 2 ). Neuronal proliferation and migration are essentially complete, while many neurons become eliminated. Yet from 80 g at mid-gestation, the mass of the brain increases to 350 g at birth, 950 g at 1 year, and 1300 to 1400 g in adulthood. This enormous increase is related to the development of an intense synaptogenesis, which results in a mild thickening but a huge tangential growth of the hemispheric cortex, thus leading to the development of the sulcation/gyration and in a spectacular brain expansion. Each neuron develops one axon only, but axons leaving their temporary connections in the subplate elongate and develop many collateral axons to reach their cortical targets, while the dendritic tree expands dramatically as well (it is estimated that at maturity, each neuron becomes connected with approximately 10,000 neurons, which means as many axonal collaterals). In the late fetal and early postnatal months, a massive increase of the number of oligodendrocytes (“myelination gliosis”) takes place, followed by an equally massive development of the myelin and of the supporting cells (astrocytes, microglia). In addition, the increasing brain diameter leads to more elongation of the axons with more myelin and more supporting tissue. The increase in volume is mostly peripheral (elongation of long-projection, commissural and association tracts; late development of the short, subcortical association tracts) while the absolute measurements of the lateral ventricular diameters remain quite stable until after birth.
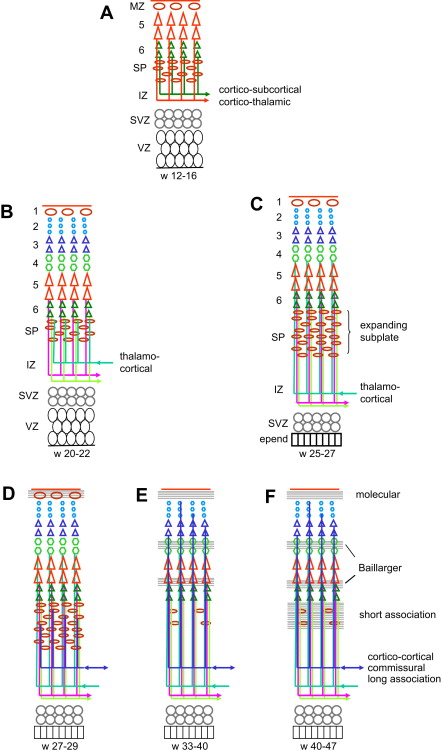
Within the cortex, the developing connectivity transforms the columnar organization into a laminar pattern. In the motor cortex the cortical neurons at 5 months (22 weeks) are still organized in columns; most early synapses are within the columns and the first horizontal connections use layer 1 to travel to other columns ; horizontal, likely afferent fibers originating from the internal capsule and from the corpus callosum are found in the deep portion of the IZ. At 7 months (about 30 weeks) the laminar pattern is better defined, especially in the deeper cortical layers, with well-defined horizontal fiber tracts in layer 1, in the developing layer 4 (future external band of Baillarger) and between layers 5 and 6 (future internal band of Baillarger). Two weeks later (32 weeks, 7.5 months), the neurons have become more mature in all layers and the horizontal stratification is well apparent (demonstrated by DTI at week 36 ). At term, the cellular maturation is complete and the horizontal pattern fully established, with afferent corticocortical fibers present in layer 3. Only the complexity of the organization changes in the following months. In the ferret and the cat, it has been shown that the lateral expansion of the cortex originates at the crown of the gyrus, corresponding to the late cellular maturation, organization, and connections there, while the bottom of the sulci would represent relatively fixed points, and apoptosis would be more important in the sulci than at the crown of the gyri.
The development of the white matter is related to the development of the cortex, and accordingly connectivity proceeds from the deeper layers (corticothalamic, corticospinal, thalamocortical) to the superficial ones (long-association and commissural tracts, then short-association tracts). The single most important structure in the development of the white matter is the SP. The SP plays the essential role of a wait zone by guiding efferent axons and establishing transient connections with efferent axons until their cortical target cells are mature enough to become connected. The SP expands markedly during the gestation, assumedly both by dispersion due to accumulating incoming axons and by the addition of new cells. It is most prominent about week 28, and especially so in the highly associative areas such as the anterior frontal cortex. SP neurons send axons to both the cortex and the subcortical structures, which serve to guide the cortical and subcortical fibers, and connect transiently with incoming fibers. The first efferent axons to leave the cortex are likely to be the corticothalamic axons from future layer 6 and the corticospinal (pyramidal) axons from future layer 5, although not much is found in the literature regarding their development in man. It should occur early because the corticothalamic fibers, originating from the deepest layer, are likely to be the first, while the corticospinal fibers are seen at the pyramidal decussation as early as 8 weeks (before the neuronal body reaches layer 5); both are likely to be guided to the internal capsule by SP neurons (see Fig. 2 A). On the other hand, the first afferent axons to approach the cortex are the thalamocortical axons: pioneer fibers are seen in the SP as early as week 12, more are seen accumulating in the superficial SP at week 22, and cortical layer 4 becomes connected by week 26 (see Fig. 2 B, C). Commissural and long-association fibers reach the SP between weeks 24 and 29 and extend to the cortex itself about 33 to 35 weeks while the SP starts receding (see Fig. 2 D, E). At the same time, the thalamocortical afferents are promoting local intracortical circuits, and short-association fibers start connecting with layers 2 and 3. The development of these short corticocortical association fibers continues until postnatal week 7 and is related to the late persistence of the SP in the high-level associative areas (see Fig. 2 F). During the first postnatal years the number of synapses increases considerably; later on, however, about age 4 to 6 years, a cortical areal specialization occurs with a corresponding pruning of the axonal branches in excess.
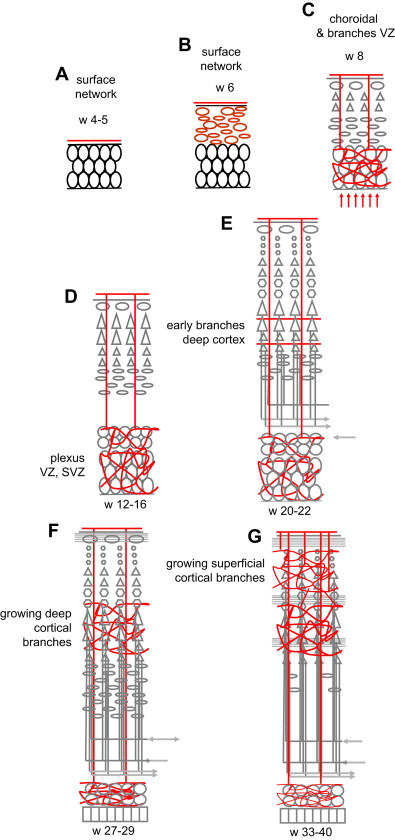
Development of Cortical Vascularization
During development, vascularization is continuously precisely adapted to the metabolic needs of the moment (see Ref. for review) ( Fig. 3 ). Vascularization evolves in 4 overlapping phases of amniotic, meningeal, choroidal, and intrinsic capillary supply. The open neural plate in week 4 is fed by diffusion from the amniotic fluid. After its closure at the end of week 4, the NT is fed by diffusion from the surrounding primitive meningeal capillaries (see Fig. 3 A, B). With the expansion of the brain vesicles and the enlargement of the ventricles, the primitive meninge invaginates into the ventricles to form the choroid plexuses: in addition to the peripheral supply from the meningeal network, oxygen, glucose, and other nutrients now diffuse from the plexus to the ventricular wall where the germinal tissue is developing; this corresponds to the preplate stage (weeks 6–7) (a major role for the choroid plexuses persists, however, as they reach their maximal size at 11 weeks ). After week 7, as the CP appears the NT becomes too thick to be fed by extrinsic diffusion alone, and the first intrinsic vessels, actually primitive sinusoid capillaries, enter the brain tissue from the periphery toward the deep germinal areas (VZ first and then SVZ) (see Fig. 3 C). These vessels cross the CP without giving it any branch, and form arteriovenous loops in the germinal zone, which expand and develop into a rich vascular plexus by 12 to 15 weeks (see Fig. 3 D). The first horizontal branches to enter the deep cortical layers appear at 20 weeks (see Fig. 3 E), and progressively become more numerous until 27 weeks (see Fig. 3 F). New short radial branches emerge from the superficial network during the last trimester to feed the superficial layers (see Fig. 3 G). The development of the cortical vascular system therefore reflects and is adapted to the progressive development of the connectivity during the second half of gestation, especially the last trimester, advancing from the deep to the superficial layers. However, this does not mean that the blood flow is significantly increased. The cerebral blood flow (CBF) measurements performed in prematures (allowing for the limitations inherent to the clinical context in such patients) seem to indicate that the CBF remains low at 10 to 20 mL/100 g/min in the last trimester until term (see Ref. for review). A steep increase of the CBF values occurs after birth, peaking at 70 mL/100 g/min at 5 years before declining to adult levels of 50 mL/100 g/min at the end of the adolescence. Surprisingly, the increase seems to occur at the same time after birth in premature and in term infants, and therefore seems to relate to the conditions of extrauterine life rather than to gestational age. These figures represent an average of the blood flows of the gray and white matter: assuming that the blood flow of the white matter does not change much over time and that white matter represents 50% of the brain in volume, the perfusion values of the gray matter in young children are still more remarkable. (Quantitative blood flow data, however, are scarce in that age range and sometimes discordant, given the fragility of the patients and the use of different technical approaches.)
Development of the cerebral cortex
Cortical Anatomy
The cerebral cortex comprises the trilayered olfactory paleocortex and hippocampal archicortex, and an extensive 6-layered neocortex (90% of the cortical surface in human). In advanced mammals and especially in humans, it is conspicuously folded, two-thirds of the cortical surface being located inside the sulci; cortical folding is related to the development of the connectivity. The sulci have been classified into primary sulci (pericallosal, cingulate, parieto-occipital, hippocampal sulci) and secondary sulci (such as the central, precentral and postcentral, intraparietal, frontal, temporal, calcarine and occipital sulci). The primary and secondary sulci may vary in shape slightly but are constant and symmetric in location. The sulci delineate the gyri, which more or less reflect the functional areas of Brodmann. The primary sulci become apparent shortly after mid-gestation, and the secondary sulci appear between 25 and 30 weeks. Tertiary sulci are branches of the primary and secondary sulci and appear mostly after birth; they are extremely variable.
The thickness of the neocortex varies from 1 to 3 mm, thinner in the depth of the sulci and thicker at the crown of the gyri. Pyramidal neurons (glutamatergic, excitatory) are the most numerous (80%) and establish long-range connections; interneurons (GABAergic, inhibitory) establish local, intracortical connections between the pyramidal neurons. The neurons are primarily organized in columnar units, but because of the intracortical course of the connecting fibers they become organized in layers. From the surface to the depth, the neocortical layers are as follows, albeit with some overlapping between them:
- •
Layer 1 or molecular layer contains mostly local connecting fibers
- •
Layer 2 receives corticocortical afferents (association and commissural fibers)
- •
Layer 3 sends corticocortical efferents (association and commissural fibers)
- •
Layer 4 or granular layer receives the corticothalamic afferents
- •
Layer 5 or pyramidal layer sends the cortico-subcortical efferents (to the striatum, brainstem, and cord)
- •
Layer 6 or polymorphic layer sends the corticothalamic efferents.
The 6-tier layering of the cortex is due to the predominantly horizontal organization of the intracortical fiber tracts. The most prominent fiber layers are in layer 1, in layer 4 (external band of Baillarger), and between layers 5 and 6 (internal band of Baillarger). If the general cortical pattern is constant, the proportion between the layers varies according to the cortical location, resulting in the various histologic patterns that characterize the cortical functional areas of Brodmann.
Formation of the Cortex
In the last 4 decades a considerable amount of information has accumulated regarding the development of the cortex, notably in the last decade (for reviews see Refs. ) ( Fig. 1 ). The early central nervous system emerges from the surface ectoderm as a band of dorsal midline neuroepithelium or neural plate during the third week (for review see Ref. ). This neural plate forms a groove with neural folds and closes to form the neural tube (NT) during the fourth week (neurulation); 3 cerebral vesicles (forebrain, midbrain, and hindbrain) become apparent during the fifth week, and the lateral evaginations of the cerebral hemispheres develop from the forebrain during the sixth week. Under the influence of ventralization and dorsalization factors, the hemispheric vesicles become divided into a basal part or subpallium (future basal ganglia) and a dorsal part or pallium (future cortex and white matter), each with its own germinal zone, the dorsal one producing pyramidal neurons and the basal one (ganglionic eminence) producing the cortical interneurons as well as the neurons of the basal ganglia (in humans interneurons may come from the pallial germinal zone as well). The division is clearly apparent during the seventh week.
Proliferation of neuroepithelial, truly neural stem cells already begins in the fourth week in the neural plate. As the NT closes, its whole thickness forms a proliferating zone where the cells divide in a symmetric way (one stem cell produces two stem cells) (see Fig. 1 A). At the end of the fifth week the proliferation process switches to asymmetric divisions (one stem cell produces one stem cell and one neuron) and the differentiated neurons accumulate at the periphery: as a consequence the wall of the NT contains a deep germinative zone, which is called the ventricular zone (VZ), and a peripheral zone with the first neurons, which is called the primordial plexiform layer or preplate (PP) (see Fig. 1 B). The distance between the ventricular and the meningeal surfaces of the NT is short in the early stages, and the differentiated cells are able to migrate by somal translocation (nucleokinesis): from the germinal zone where they are born they extend a process toward the meningeal surface, the nucleus migrates into this process toward the surface while the ventricular process shortens and loses its ventricular contact. One of the genes that controls this nucleokinesis is the LIS1 gene, whose defect is associated with one major form of lissencephaly. The process of translocation is used by the neurons of the PP and possibly by the early neurons (future layer 6) of the cortical plate (CP). The PP contains Cajal-Retzius cells and other neurons that are the first to establish extracortical connections. When the cortical plate appears on about day 50 (end of week 7), it divides the PP into two layers: the superficial layer or marginal zone (MZ) contains the reelin-positive Cajal-Retzius cells (in addition to various other neurons), and the subcortical layer forms the subplate (SP) and contains reelin-negative neurons (see Fig. 1 C). Cajal-Retzius cells play a major role of controlling the migration of the neurons in the CP; the subplate is essential also, as it directs outgoing axons and maintains transient connections with the incoming axons until the cortex becomes ready.
Radial migration of glutamatergic pyramidal neuron to the CP begins at the end of week 7 in the lateral part of the telencephalon, and a week later in its posteromedial aspect; the peak migratory activity lasts until mid-gestation (weeks 20–22) and migration is essentially complete before the third trimester. Radial migration uses specialized cells, the radial glia, to guide the pyramidal neuron from the germinal zone to the CP. Each radial glial cell has a process anchored on the ventricular surface, and a radial process that extends to the pial basement membrane (where it often makes contact with vessels ), so that the radial glia forms a scaffolding across the mantle. The newly generated neurons travel perpendicular to the surface, from the pallial VZ along the glial fibers to the CP, where they are induced to detach from the radial glia by the signal (Reelin) provided by the Cajal-Retzius cells. As a consequence early-migrating cells are in the deep cortical layers and late-arriving cells are close to the surface: this is called the inside-out pattern (see Fig. 1 C–E). A first wave of migration toward the deep layer 6 develops at about 7 to 11 weeks; a second wave toward layer 5 occurs at about 12 to 16 weeks; a third and last wave to the more superficial layers occurs after 16 weeks. This last wave is prominent in primates, including man, and corresponds to the neurons that will develop corticocortical connections. Late migration of individual neurons may continue even after the end of the cellular proliferation until after birth. As a consequence of the radial migration, all neurons using a single radial cell form a single column in the CP. The glia-guided migration depends on the cellular microfilament network, and involves the FLN1 gene (whose defect has been demonstrated in specific varieties of gray matter heterotopia), as well as on two proteins associated with the regulation of the actin network, Cdk5 and p35. Doublecortin (DCX) also is involved with radial radiation, and defects of the DCX gene are associated with some human lissencephalies (for review see Ref. ).
Besides their role of guidance, the radial glia recently have been shown to be stem cells and to produce neuronal progenitors. In a rather complex process, they divide asymmetrically in the VZ, producing another radial glial cell and a neuronal progenitor. The neuronal progenitor moves to the subventricular zone (SVZ) (phase 1) where it stays for up to 24 hours, becomes multipolar, and establishes multiple cellular contacts, moves tangentially free from radial glial attachment, and becomes dispersed within the SVZ before dividing symmetrically (phase 2). The new neurons may migrate directly to the cortex along the radial glia, but most translocate back to the VZ (phase 3), from where they make their final journey toward the CP along the radial glial fibers (phase 4).
Tangential migration of the GABAergic inhibitory interneurons occurs in close association with the radial migration of the pyramidal neurons (the first interneurons are seen at about weeks 6–7, even before the appearance of the CP ). These neurons typically originate in the ventral VZ of the medial ganglionic eminence (MGE; the primordium of the globus pallidus ) and travel parallel to the surface of the hemisphere toward the pallium. Like the radial migration, the tangential migration is complex (for review see Ref. ). Studies in rodents show that there are primarily two migration streams, one along the MZ and the other along the deep IZ/SVZ (the intermediate zone [IZ] is the portion of the pallium that is located between the SP and the germinal SVZ). From their MGE origin, some interneurons disperse into the IZ/SVZ before reaching the CP either radially or obliquely; some travel along the MZ and enter the CP from above; some travel in the IZ/SVZ, then reach the MZ radially across the CP, disperse into the MZ, and enter the CP from above. However, the majority of interneurons (70%) travel through the IZ/SVZ and dive toward the ventricle to enter the VZ, where they pause before resuming their course and migrating radially to the CP. In this process, the interneurons are likely to acquire laminar address information, possibly mediated by GABA information. The partial convergence during their migration of at least some pyramidal neurons and interneurons might allow transmission of positional information. Pyramidal neurons pause in the SVZ, become multipolar, and may contact interneurons there. The different migration speed—10 μm/h for the radial migration, 50 μm/h for the tangential ones—may favor birth-date related encounters, and cellular birth date relates to laminar position precisely. What guides the interneurons—guiding glia, axons projecting from the CP—is not clear, but the process has been shown to involve class 3 semaphorins, neuropilins, cell-adhesion molecules, neuroregulins, and the slit/robo complex.
The organization of the pallium changes and becomes more complex as it develops ( Table 1 ), and so does the terminology (see Fig. 1 ). In weeks 4 to 5 the pallium is a simple homogeneous pseudostratified neuroepithelium. Between week 5 and week 7 (before the appearance of CP) it comprises a deep germinal zone VZ and a superficial postmitotic zone PP. After the CP divides the PP during week 8, the postmitotic zone is made up of 3 layers (MZ, CP, and SP), the germinal zone is made up of 2 layers (VZ and SVZ), and an IZ in between contains migrating cells, radial glia processes, and early incoming and outgoing axons. After peaking before mid-gestation, migration stops at about 25 to 27 weeks. The radial glia loses contact with the ventricle, migrates toward the cortex, and forms astrocytes (changing its nestin and PAX6 expression for glial fibrillary acidic protein). The pallial VZ disappears leaving the unicellular layer of ependyma only, but the SVZ persists and contains stem cells even in the adult brain, presumably a potential source of brain tumor cells. The germinal zone of the lateral ganglionic eminence remains prominent for some time (the so-called germinal matrix of the premature brain), before vanishing progressively during the last prenatal weeks. After a peak of complexity between 18 and 28 weeks, the cellularity of the MZ, notably the Cajal-Retzius cells, regresses and disappears before term. On the contrary, the SP expands significantly until the third trimester, being largest at week 28, particularly under the frontal associative cortex, and then attenuates until about term, leaving interstitial neurons only in the white matter. As connectivity develops, white matter fibers progressively invade both the IZ and the SP area, which together form the final hemispheric white matter.
| Age in Weeks | Forebrain | Cellular Processes | Organization of Pallium | White Matter | Metabolic Supply/Vasculature |
|---|---|---|---|---|---|
| 3 | Neural plate | Stem cell proliferation | Pseudostratified | — | Amniotic fluid |
| 4 | Anterior neural plate neural tube closure | Stem cell proliferation | Pseudostratified | — | Primitive meninges |
| 5–6 | Prosencephalon then hemispheres (pallium/subpallium) | First peripheral primordial neurons | VZ and PP | — | Choroid plexus |
| 7–9 | Pallium | Translocation First wave to layer 6 | Postmitotic: MZ, CP, SP Germinal: VZ | Corticothalamic Corticospinal | Choroid plexuses First perforators to VZ/MGE |
| 10–12 | Pallium | Radial glia Progenitors Second wave to layers 5–4 | Postmitotic: MZ, CP, SP Intermediate: IZ Germinal: VZ–SVZ | First thalamocortical in SP | Choroid plexuses Rich germinal plexus SVZ/VZ/MGE |
| 16–20 | Pallium | Third wave to layers 3–2 | — | Thalamocortical in SP Commissural and association in SP | Rich germinal plexus SVZ/VZ/MGE |
| 22–26 | Pallium Primary and early secondary sulci | End of migration and of radial glia | Disappearance of VZ Ependyma | Thalamocortical in layer 4 Early commissural and association to layers 2–3 | Rich germinal plexus SVZ/MGE Early branches in deep cortex |
| 27–32 | Pallium Secondary sulci | — | Reaches mature appearance Prominent SP | Layer 1 and Baillarger Commissural and association to layers 2–3 | Germinal plexus recedes in SVZ Growing vasculature in deep cortex |
| 33–40 | Pallium Developing tertiary sulci | — | SP recedes White matter in IZ/SP | Short association to layers 2–3 | Radial vasculature in superficial cortex End of germinal plexus SVZ/MGE |
| 42–47 | Pallium Developing tertiary sulci | — | End of frontal SP | Short association to layers 2–3 | Steep increase of CBF |
Cellular apoptosis cannot be dissociated from proliferation and organization. The number of neurons in the brain peaks at week 28, but as many as 50% die through apoptosis before the end of the adolescence. Two main periods of apoptosis occur prenatally. The first lasts from week 7 to week 13, and involves proliferating progenitors and young neurons in the VZ. The second, regulated by synaptic activity, cellular contacts, and glial and neuronal trophic factors, eliminates neurons within the CP itself between week 19 and week 23.
Cortical Organization and Developing Connectivity
The period after 22 weeks is the period of organization and differentiation of the cortex ( Fig. 2 ). Neuronal proliferation and migration are essentially complete, while many neurons become eliminated. Yet from 80 g at mid-gestation, the mass of the brain increases to 350 g at birth, 950 g at 1 year, and 1300 to 1400 g in adulthood. This enormous increase is related to the development of an intense synaptogenesis, which results in a mild thickening but a huge tangential growth of the hemispheric cortex, thus leading to the development of the sulcation/gyration and in a spectacular brain expansion. Each neuron develops one axon only, but axons leaving their temporary connections in the subplate elongate and develop many collateral axons to reach their cortical targets, while the dendritic tree expands dramatically as well (it is estimated that at maturity, each neuron becomes connected with approximately 10,000 neurons, which means as many axonal collaterals). In the late fetal and early postnatal months, a massive increase of the number of oligodendrocytes (“myelination gliosis”) takes place, followed by an equally massive development of the myelin and of the supporting cells (astrocytes, microglia). In addition, the increasing brain diameter leads to more elongation of the axons with more myelin and more supporting tissue. The increase in volume is mostly peripheral (elongation of long-projection, commissural and association tracts; late development of the short, subcortical association tracts) while the absolute measurements of the lateral ventricular diameters remain quite stable until after birth.
Within the cortex, the developing connectivity transforms the columnar organization into a laminar pattern. In the motor cortex the cortical neurons at 5 months (22 weeks) are still organized in columns; most early synapses are within the columns and the first horizontal connections use layer 1 to travel to other columns ; horizontal, likely afferent fibers originating from the internal capsule and from the corpus callosum are found in the deep portion of the IZ. At 7 months (about 30 weeks) the laminar pattern is better defined, especially in the deeper cortical layers, with well-defined horizontal fiber tracts in layer 1, in the developing layer 4 (future external band of Baillarger) and between layers 5 and 6 (future internal band of Baillarger). Two weeks later (32 weeks, 7.5 months), the neurons have become more mature in all layers and the horizontal stratification is well apparent (demonstrated by DTI at week 36 ). At term, the cellular maturation is complete and the horizontal pattern fully established, with afferent corticocortical fibers present in layer 3. Only the complexity of the organization changes in the following months. In the ferret and the cat, it has been shown that the lateral expansion of the cortex originates at the crown of the gyrus, corresponding to the late cellular maturation, organization, and connections there, while the bottom of the sulci would represent relatively fixed points, and apoptosis would be more important in the sulci than at the crown of the gyri.
The development of the white matter is related to the development of the cortex, and accordingly connectivity proceeds from the deeper layers (corticothalamic, corticospinal, thalamocortical) to the superficial ones (long-association and commissural tracts, then short-association tracts). The single most important structure in the development of the white matter is the SP. The SP plays the essential role of a wait zone by guiding efferent axons and establishing transient connections with efferent axons until their cortical target cells are mature enough to become connected. The SP expands markedly during the gestation, assumedly both by dispersion due to accumulating incoming axons and by the addition of new cells. It is most prominent about week 28, and especially so in the highly associative areas such as the anterior frontal cortex. SP neurons send axons to both the cortex and the subcortical structures, which serve to guide the cortical and subcortical fibers, and connect transiently with incoming fibers. The first efferent axons to leave the cortex are likely to be the corticothalamic axons from future layer 6 and the corticospinal (pyramidal) axons from future layer 5, although not much is found in the literature regarding their development in man. It should occur early because the corticothalamic fibers, originating from the deepest layer, are likely to be the first, while the corticospinal fibers are seen at the pyramidal decussation as early as 8 weeks (before the neuronal body reaches layer 5); both are likely to be guided to the internal capsule by SP neurons (see Fig. 2 A). On the other hand, the first afferent axons to approach the cortex are the thalamocortical axons: pioneer fibers are seen in the SP as early as week 12, more are seen accumulating in the superficial SP at week 22, and cortical layer 4 becomes connected by week 26 (see Fig. 2 B, C). Commissural and long-association fibers reach the SP between weeks 24 and 29 and extend to the cortex itself about 33 to 35 weeks while the SP starts receding (see Fig. 2 D, E). At the same time, the thalamocortical afferents are promoting local intracortical circuits, and short-association fibers start connecting with layers 2 and 3. The development of these short corticocortical association fibers continues until postnatal week 7 and is related to the late persistence of the SP in the high-level associative areas (see Fig. 2 F). During the first postnatal years the number of synapses increases considerably; later on, however, about age 4 to 6 years, a cortical areal specialization occurs with a corresponding pruning of the axonal branches in excess.
Development of Cortical Vascularization
During development, vascularization is continuously precisely adapted to the metabolic needs of the moment (see Ref. for review) ( Fig. 3 ). Vascularization evolves in 4 overlapping phases of amniotic, meningeal, choroidal, and intrinsic capillary supply. The open neural plate in week 4 is fed by diffusion from the amniotic fluid. After its closure at the end of week 4, the NT is fed by diffusion from the surrounding primitive meningeal capillaries (see Fig. 3 A, B). With the expansion of the brain vesicles and the enlargement of the ventricles, the primitive meninge invaginates into the ventricles to form the choroid plexuses: in addition to the peripheral supply from the meningeal network, oxygen, glucose, and other nutrients now diffuse from the plexus to the ventricular wall where the germinal tissue is developing; this corresponds to the preplate stage (weeks 6–7) (a major role for the choroid plexuses persists, however, as they reach their maximal size at 11 weeks ). After week 7, as the CP appears the NT becomes too thick to be fed by extrinsic diffusion alone, and the first intrinsic vessels, actually primitive sinusoid capillaries, enter the brain tissue from the periphery toward the deep germinal areas (VZ first and then SVZ) (see Fig. 3 C). These vessels cross the CP without giving it any branch, and form arteriovenous loops in the germinal zone, which expand and develop into a rich vascular plexus by 12 to 15 weeks (see Fig. 3 D). The first horizontal branches to enter the deep cortical layers appear at 20 weeks (see Fig. 3 E), and progressively become more numerous until 27 weeks (see Fig. 3 F). New short radial branches emerge from the superficial network during the last trimester to feed the superficial layers (see Fig. 3 G). The development of the cortical vascular system therefore reflects and is adapted to the progressive development of the connectivity during the second half of gestation, especially the last trimester, advancing from the deep to the superficial layers. However, this does not mean that the blood flow is significantly increased. The cerebral blood flow (CBF) measurements performed in prematures (allowing for the limitations inherent to the clinical context in such patients) seem to indicate that the CBF remains low at 10 to 20 mL/100 g/min in the last trimester until term (see Ref. for review). A steep increase of the CBF values occurs after birth, peaking at 70 mL/100 g/min at 5 years before declining to adult levels of 50 mL/100 g/min at the end of the adolescence. Surprisingly, the increase seems to occur at the same time after birth in premature and in term infants, and therefore seems to relate to the conditions of extrauterine life rather than to gestational age. These figures represent an average of the blood flows of the gray and white matter: assuming that the blood flow of the white matter does not change much over time and that white matter represents 50% of the brain in volume, the perfusion values of the gray matter in young children are still more remarkable. (Quantitative blood flow data, however, are scarce in that age range and sometimes discordant, given the fragility of the patients and the use of different technical approaches.)
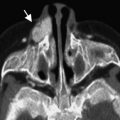
Focal cortical dysplasia
Definition and Classification of FCD
In 1971, Taylor and Falconer with Bruton and Corsellis published a series on 10 epileptic patients, in which the parts of the brain where the abnormal electrical activity existed were surgically removed. The investigators noted histologic abnormalities, which lacked the tumor-like expansion of hamartomata and which, for that reason, they called “a particular form of localized cortical dysplasia.” The lesions were characterized histologically by “congregations of large, bizarre neurons which were littered through all but the first cortical layer” with “in most but not all cases, grotesque cells, probably of glial origin, [which] were also present in the depth of the affected cortex and in the subjacent white matter.” The study noted that in all cases the brain surface was normal with no tuber-like appearance; when cut, the cortex looked macroscopically normal in 7 of 10 cases but was wide with blurred junction with the white matter in 3 of 10 cases. Microscopically there was disorganization of the laminar architecture with large aberrant neurons scattered randomly, which stood out by their number, large size, and bizarre structure, and the common presence (7 of 10 cases) of large, multinucleated cells in the deep cortical layers and the white matter. There were reactive astrocytes but no fibrous gliosis, and sometimes a reduction of the myelinated fibers in the underlying white matter. The investigators mentioned previous reports of findings somewhat similar to theirs, that had been related to formes frustes of tuberous sclerosis, only to mention that their patients did not present the features, either clinical (family history, intellectual delay, other stigmata), radiologic (subependymal nodules, calcification), or histologic (tuber appearance, cellular depopulation), which characterize tuberous sclerosis.
From the perspective of epilepsy surgery in children this article, which defined the entity “focal cortical dysplasia (FCD),” was probably the most seminal in the past half-century. FCD is the single most important cause of focal refractory epilepsy in children, histologically proved in almost 50% of children undergoing epilepsy surgery (20% of adults). With time, it became obvious that it was a heterogeneous entity, with distinct clinical and imaging characteristics, and different surgical responses, the surgical indications and the prognosis being closely related with the pathologic subtype, so that classifications were proposed to provide more consistency. The classification most widely used until recently (“Cleveland” or Palmini classification ) was proposed in 2004 by a panel of experts. It made a clear distinction between the architectural abnormalities (dyslamination, heteropic and misoriented neurons) and the cytologic abnormalities (giant neurons, immature neurons, dysmorphic neurons, balloon cells), with the following 3-tiered classification (from the milder to the more severe histologic changes).
- •
Mild malformations of cortical development (mMCD) are characterized by ectopic neurons located in (type I) or outside (type II) layer 1 (this subgroup replaced the previous subgroup of microdysgenesis). Cases of mMCD were assumed not to be detectable by the then current MR techniques.
- •
FCD type I are characterized by architectural abnormalities (dyslamination of the cortex without or with additional features of mMCD), which may be isolated (type IA) or associated with giant or immature neurons (type IB).
- •
FCD type II are characterized by the presence of monstrous cells in addition to the architectural abnormalities, either dysmorphic neurons only (type IIA) or dysmorphic neurons and balloon cells (type IIB). FCD type II are often referred to as Taylor-type FCD.
This classification has 3 main limitations, the first being that such a grading system suggests different degrees of severity in the same disease entity. The second limitation was revealed by a blinded evaluation of interobserver and intraobserver reproducibility of histologic diagnosis in mMCD-FCD (26 specimens rotated among 8 neuropathologists), which showed that whereas the reproducibility rate was high for FCD type II (especially IIB), it was quite low for mMCD and FCD type I, meaning that except for the monstrous cells of type II, the other subgroups lack an unequivocal morphologic definition. The third limitation is that the classification did not take into account the FCD associated with other disorders including obviously acquired diseases such as sequelae of perinatal hypoxic ischemic encephalopathy, trauma, infection, or strokes. For these reasons, a more refined classification is proposed by the Diagnostic Method Commission of the International League Against Epilepsy (ILAE) (which includes the Cleveland classification experts as well). Considering the FCD only (the mMCD is evaluated later), this new classification retains the group of FCD type IIA and type IIB, as it is clearly defined by the monstrous dysmorphic neurons and balloon cells. It better defines type I and introduces a new type III FCD, associated with another, principal lesion.
- •
FCD type I (isolated)
- ○
Type IA: abnormal radial cortical lamination
- ○
Type IB: abnormal tangential cortical lamination
- ○
Type IC: abnormal radial and tangential lamination
- ○
- •
FCD II
- ○
Type IIA: dysmorphic neurons
- ○
Type IIB: dysmorphic neurons and balloon cells
- ○
- •
FCD III (associated with principal lesion)
- ○
Type IIIA: abnormal temporal cortical lamination associated with hippocampal sclerosis (HS)
- ○
Type IIIB: abnormal cortical lamination adjacent to a glial or glioneuronal tumor
- ○
Type IIIC: abnormal cortical lamination adjacent to a vascular malformation
- ○
Type IIID: abnormal cortical lamination adjacent to any other lesion acquired during early life (eg, trauma, ischemia, infection).
- ○
Note that should an FCD type II be associated with another lesion, it would not be considered a subgroup of FCD III but the association of two principal lesions. Also, it is proposed that by convention the use of the term “dual pathology” be restricted to cases of HS presenting with a second principal lesion of the brain (tumor, vascular malformation, glial scar, encephalitis, MCD including FCD type II), even outside the ipsilateral temporal lobe.
As is implicit in the classification, FCD are pathogenetically heterogeneous. FCD type II is characterized by monstrous cells and is assumed to be truly developmental (this is supported by the similarities between FCD type II and TSC brain lesions). The dysmorphic neurons may present with either a pyramidal or an interneuronal phenotype, so that apparently the cellular dysplasia may affect different cellular lineages. The balloon cells are consistent with dysplastic glia, which is the same lineage as the pyramidal dysmorphic neurons. White matter changes may be mild or severe, and have been related to a heterotopic distribution of dysplastic cells and neurons along the path of migration, a lack or loss of myelin, inflammation, oligodendrocytic satellitosis, and astrocytosis. In the classification of MCD, FCD type II accordingly is understood as a defect of proliferation/apoptosis. Moreover, there is some evidence that it could result from an excessive neurogenesis and retention of preplate cells in the late phases of late corticoneurogenetic (first half of second trimester). FCD type I and mMCD are classified as organization disorders : abnormal retention of the radial cortical pattern, lack of tangential lamination, and giant and immature neurons may relate to a true dysplasia or to a disruption of the normal cortical development occurring as late as during the neonatal period.
Imaging of FCD
In clinical situations, FCD is the most difficult MCD to diagnose with MR imaging. Various strategies have been suggested over the years to improve the detection rate of the lesion using MR, electroclinical data, and complementary functional/metabolic imaging modalities. As a rule, the less severe the dysplastic changes, the more likely the MR is to appear normal. The diagnostic efficacy obviously depends on better equipment (high magnetic fields, multiple phased array coils) and greater expertise of the neuroradiologist.
FCD type II presents the most characteristic appearances ( Figs. 4–8 ) : an increased cortical thickness with a blurring of the cortical-subcortical junction (see Fig. 4 ); a high T2/FLAIR signal of the cortex; a low T1, high T2/FLAIR signal in the subcortical white matter (see Fig. 5 ), sometimes tapering from the cortex to the ventricular wall, so reproducing the migration path (this “transmantle dysplasia” is practically pathognomonic of a FCD type IIB ) (see Fig. 6 ). The lesion may be small, sulcal, centered at the very bottom of a sulcus that is deeper than its contralateral counterpart ; there it may appear as a focal, strictly cortical clear-cut bright T1 signal (see Fig. 7 ). The lesion may extend along the walls of the sulcus, or may affect the crown of the gyrus, or the whole gyrus between 2 sulci; the gyrus then may be somewhat bulky. It may uncommonly mimic a tuber (see Fig. 8 ). Large FCD type II may also be multigyral or lobar, usually with an abnormal gyration pattern. It may rarely also be multiple, unilateral or bilateral. When large FCD type II lesions are so large as to occupy a large part of the hemisphere, they are referred to as partial, or lobar, hemimegalencephalies (HME). FCD type IIB presents only rarely without any MR abnormalities (4%); this occurs more often with FCD type IIA (22%). However, as mentioned earlier, a normal-appearing white matter may reflect mild pathologic changes with increased diffusivity and decreased fat attenuation only.