26
HYPERTHERMIA
SHARVARI DHARMAIAH, VINAY RAO, AND JENNIFER S. YU
Question 1
What is hyperthermia?
Question 3
What are the different methods of regional hyperthermia?
Question 1 What is hyperthermia?
Answer 1
Hyperthermia is a form of cancer treatment wherein body tissues are exposed to ablative (50°C–60°C) or fever range (39°C–43°C) temperatures. Hyperthermia improves cancer control through multiple mechanisms including directly damaging or killing cancer cells, increasing perfusion to improve radiation sensitivity and drug delivery, and improving the antitumor immune response. It is most often used alongside other forms of cancer therapy, such as radiation and chemotherapy.
Chu K, Dupuy D. Thermal ablation of tumors: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
Hall EJ, Giaccia AJ. Hyperthermia. In: Hall EJ, Giaccia AJ, eds. Radiobiology for the Radiologist. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:490–511.
Question 3 What are the different methods of regional hyperthermia?
Answer 3
In regional hyperthermia, various methods are used to heat large areas of the body such as a limb, body cavity, or an organ. The different approaches utilized are deep tissue, regional perfusion, or continuous hyperthermic peritoneal perfusion (CHPP) techniques. Deep tissue methods are used to treat tumors within the body where external applicators are positioned around the target organ or cavity and radiofrequency or microwave energy is administered. Regional perfusion is used to treat tumors in the arms and legs or cancers in some internal organs. In this method, which is often combined with chemotherapy, a portion of the patient’s blood is removed, heated, and then perfused through the limb or organ. CHPP is used to treat cancers within the peritoneal cavity. During surgery, the treatment is administered through the use of heated anticancer drugs that flow from a warming device through the peritoneal cavity.
Hall EJ, Giaccia AJ. Hyperthermia. In: Hall EJ, Giaccia AJ, eds. Radiobiology for the Radiologist. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:490–511.
van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13(8):1173–1184.
Question 4
In in vitro models, what is the relationship between temperature and cell death?
Question 6
How is the Arrhenius plot useful in assessing the effects of thermal damage in tissues?
Question 4 In in vitro models, what is the relationship between temperature and cell death?
Answer 4
In in vitro models, cell death occurs in an exponential pattern that can be modeled with the rate of killing increasing as the temperature increases.
Hall EJ, Giaccia AJ. Hyperthermia. In: Hall EJ, Giaccia AJ, eds. Radiobiology for the Radiologist. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:490–511.
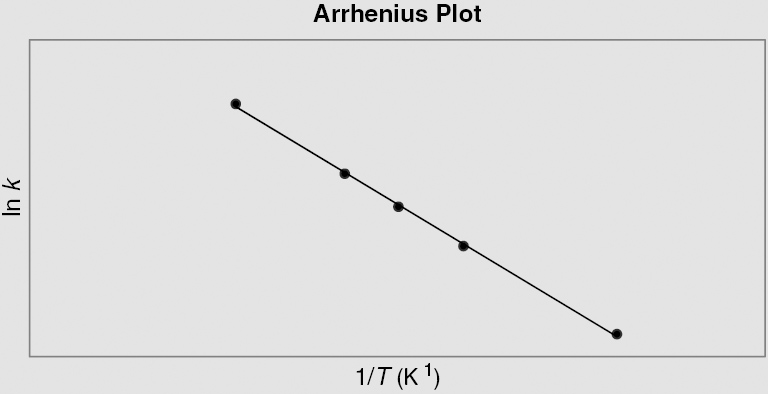
Question 6 How is the Arrhenius plot useful in assessing the effects of thermal damage in tissues?
Answer 6
The Arrhenius plot serves as a basis for understanding the thermal doses necessary in clinical hyperthermia applications. In an Arrhenius plot, the slope designates the activation energy (Ea) of the chemical process involved in killing cancer cells. Above the “breakpoint,” a temperature point (about 43°C) in which there is a significant change in the slope of the plot, a change in 1°C signifies a doubling of the rate of cell killing. Below this value, the rate of cell death can drop by a factor between 2 and 4 for each degree Celsius drop. Differences in activation energy both below and above this breakpoint may demonstrate different means of cell killing or the development of thermotolerance in some cells.
The Arrhenius equation is described by ln k = ln A – Ea/RT, where k is the rate constant, T is the temperature in Kelvin, A is a preexponential factor, Ea is the activation energy, and R is the universal gas constant. The y-intercept = ln A and slope = – Ea/R.
Hall EJ, Giaccia AJ. Hyperthermia. In: Hall EJ, Giaccia AJ, eds. Radiobiology for the Radiologist. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:490–511.
Question 7
What is a thermal enhancement ratio (TER)?
Question 9
What is the therapeutic gain factor?
Question 7 What is a thermal enhancement ratio (TER)?
Answer 7
TER is the ratio of radiation doses with and without the administration of heat necessary to produce a specific level of biological damage. The TER increases with increasing temperature and can be used to predict effective treatment doses. TER from clinical data ranges from 1.15 to 1.5.
Hall EJ, Giaccia AJ. Hyperthermia. In: Hall EJ, Giaccia AJ, eds. Radiobiology for the Radiologist. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:490–511.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree