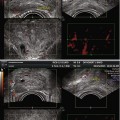
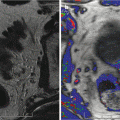
Fig. 16.1
Prostate cancer subject treated with β-sit/AOX supplement program. (a) DCE-MRI of Gleason 8 diffuse prostate cancer (vascular enhancement is red). (b) Posttreatment scan of same area showing marked reduction of vascular enhancement
The results shown here illustrate both the efficacy of restoring fundamental nutritional support that has been systematically removed from our diets by alterations in the food supply, and the need for a means to monitor, on a regular basis, each individual’s response to treatment. Given the number of factors involved in nutritional support, this is the only way to know that this individual’s body is capable of restoring its anticancer functions. The same monitoring approach can be applied to any therapeutic intervention, especially since the 3-D sonogram is not compromised by the treatment itself.
When establishing a monitoring schedule for an individual, two additional areas need to be kept in mind, interval cancers and evaluating successful treatment.
Interval Cancers
The concept of fast-growing cancers called “interval cancers” has led to routine biannual screening of male and female high-risk patients. It is recognized that mammography misses invasive breast cancers with great frequency, so there is a half-year time period in which health-conscious women should alert themselves to the possibility of early breast cancer as they routinely undergo ultrasound breast screening twice a year. In practice, about 5 % of men develop aggressive interval cancers within half a year from their last normal or stable evaluation. A presentation entitled “Interval Cancers Of The Prostate: Evaluation By 3-T MRI And 3-D Power Doppler Ultrasound” was made at the 2011 meeting of the Societe Francaises de Radiologie in Paris, demonstrating that new aggressive tumors may occur more rapidly than clinically expected and may, in part, explain the failure of certain treatments (Bard 2011).
When a man has not had a biopsy or has had a negative biopsy and a vascular tumor is demonstrated on the 3-D PDS, an MRI exam is recommended, which shows the prostate gland, the capsule of the prostate, the regional lymph glands, seminal vesicles, and boney pelvis. Other bones, to which cancer frequently spreads such as the lower spine and hip, may also be imaged for abnormalities. While the MRI exam is not as good an indicator of cancer aggression, it shows spread of the tumor outside the prostate capsule to the lymph nodes better than the 3-D PDS and better than the CT scan, which is currently used as the standard test for staging.
Assessing Response to Prostate Cancer Therapy
The value of medical imaging is to:
Localize volume of disease including extracapsular extension
Assess bone metastases, seminal vesicle invasion, and lymphadenopathy
Estimate degree of aggressivity (similar to Gleason grading)
Determine efficacy of therapy
Monitor changes
Tailor future therapy
Determine tumor recurrence
Identify new tumor
One of the problems of evaluating cancer treatment is that the tumor may be rendered harmless or even dead but the volume of the tumor remains the same or even enlarges. That is, the cancer cells may be killed off and scar tissue replaces the dead cells, leaving the size of the original malignancy unchanged, or edema and necrotic fluid buildup enlarges the region, simulating tumor growth. This lesson was learned 19 years ago in treating liver tumors. The therapy would render the cancer harmless, but the size of the mass on the isotope scans, sonogram, CT, and MRI would remain unchanged or even enlarge. The same is true of some prostate cancers that are inactivated but still feel like cancer on the digital rectal exam and show a mass effect on the sonogram and MRI. There needs to be a way to monitor changes and determine the efficacy of treatment. Fortunately, the blood flows in malignancies that have been inactivated decrease or disappear and can be quickly and accurately measured in the moment. Thus, with 3-D PDS, there is a simple tool to quantify blood flow patterns to demonstrate therapeutic response.
16.8 Hormonal Treatment Options for Prostate Cancer
Testosterone has not been implicated as the cause of prostate cancer. Environmental and dietary factors are likely major contributors of the development of aggressive, invasive cancers. Despite these factors, androgens—testosterone and DHT—are thought to be the major source of PCA growth. Yet androgen ablation therapy, ABT, is a failed approach in many cases. Over time, most prostate cancer cell lines become more and more resistant to androgen receptor activities or growth suppression due to the development of androgen resistant cell lines. Initial suppression by ABT is frequently followed by rapid progression of poorly differentiated, hormone-independent cell lines within 12–36 months. Newer approaches have aimed at increasing the antiandrogen blockade. However, since androgens have significant systemic benefits, this treatment approach can have serious progressive adverse effects and QOL decrements. These antiandrogen approaches or adjunctive chemotherapy may extend the remission temporarily, but all these options have highly significant adverse effects associated with prolonged use and few individuals are able to achieve full remissions. Those cases that seem to achieve stable “remissions” have low-grade cell types that might do better with intermittent hormone blockade or alternative approaches. The primary focus on the role of androgens in the growth, progression, and suppression of PCA cell lines has limited the development of other potential endocrine approaches. Still there are no good options available when ABT failures occur.
16.8.1 The Role of Estrogens and Estrogen Receptors
One area that is reemerging is the newer scientific understanding of the role of estrogens in regulating PCA growth and progression as well as inhibition and induction of apoptosis. Earlier use of potent estrogens, such as diethylstilbestrol, were associated with some success but also serious side effects that resulted in switching to newer approaches, including androgen deprivation therapy (ADT).
The majority of PCA cell lines, including those that have developed androgen resistance after ADT, have active estrogen receptors as modulators of cell growth and apoptosis. This option opens up various newer interventions with the use of various estrogens, phytoestrogens, or SERMs with selective estrogen receptor effects. These approaches have been greatly underutilized or lacked popularity due to past side effects of high doses of oral estrogens.
At the ages we begin to see PCA becoming more prevalent/active, after age 50, there is an upward shift in estrogen production within the prostate and a downward shift in androgens, DHEA, and testosterone. Intraprostatic hormone levels do not necessarily parallel serum levels due to local intracrine production.
There is a well-described forward feedback loop that involves increased aromatase activity that results in increased estrogen production. Increased estrogen stimulates pro-inflammatory cytokines and prostaglandins that further stimulate aromatase activities and higher intraprostatic estrogen levels. This has been well described in association with intracrine estrogen production in endometriosis, uterine fibroids, and in breast, ovarian, and endometrial cancers. Local intra-tumoral aromatase activity may be greatly increased over normal tissue levels, resulting in huge increases in estrogen production by cancer cells.
What is now becoming better appreciated is the role of estrogens and estrogen receptors in regulating growth or suppression in many tissues throughout the body as well as within the prostate. Estrogens activate specific estrogen receptors, ERa and ERb, which have opposing effects on promotion of growth or inhibition of growth, control of inflammation, and induction of apoptosis within various tissues, including the prostate. ERa has pro-growth, pro-inflammatory, and antiapoptotic effects. ERb has the opposite effects and might be viewed as an anticancer receptor activating cell cycle arrest and apoptosis. Increased intraprostatic estrogens and ERa activation may play important roles in the development of BPH.
Genetic changes within cancer cells may increase or reduce ERa and ERb that may result in positive benefits or failures with estrogen treatments. In PCA, predominance of ERa over ERb results in pro-cancerous changes, growth, cell cyclic activation, and inflammation. Increased intraprostatic estradiol production leads to a vicious cycle upward that may promote cancer cell activities. So it appears that estrogens are part of the problem or part of the resolution depending on the ERa/ERb activities. The newer approaches are aimed at decreasing ERa activities and shifting to ERb dominance. Interestingly, most plant estrogens, phytoestrogens, have greater ERb activation than ERa that should reduce cancer cell activities. In fact, countries that have predominantly vegan diets have very low prostate cancer rates.
Another interesting aspect of testosterone/DHT metabolism is that one of the major metabolites of DHT, 3 Adiol, is primarily an ERb activator with suppressive cancer effects. 5AR inhibitors that suppress DHT will also suppress 3 Adiol. This leads lack of ERb effects, such as apoptosis, and results in gradual promotion of more aggressive, undifferentiated cell lines as has been reported with long-term use of 5AR inhibitors.
16.8.2 New Approach to Prostate Cancer Utilizing Estrogen Receptors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree