Breast MRI has become an integral component in breast imaging. Indications have become clearer and better defined. Guidelines and recommendations are evolving and many are recognized and published. Future applications are exciting and may possibly improve our ability to diagnose breast cancer, improving the patient’s treatment options and ultimately patient outcome.
Breast MRI has become an integral and necessary component of any breast imaging practice. The performance and clinical uses of breast MRI are now standardized and much more defined than they were several years ago. In the past few years, great strides have been made by societies in the realm of defining indications and findings on breast MRI. The most important development, however, of the past few years was made in the area of breast intervention: new biopsy coils and a choice of MR-compatible biopsy needles are now available, making percutaneous biopsy of a suspicious MR lesion a possibility. Additionally, more imaging sequences are now available from manufacturers with an increase in both image quality and speed of acquisition. Breast MRI is now available in many practices and is one of the fastest growing areas in radiology. In fact, many of our current algorithms in the detection and treatment of breast cancer have been changed by the availability of breast MRI.
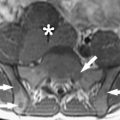
The basic strength of breast MRI lies in the detection of cancer that is occult on conventional imaging such as mammography and sonography. Many studies have shown that breast MRI is best used in situations where there is a known cancer, suspected cancer, or a high probability of finding cancer. For example, in the preoperative evaluation of the patient with a known cancer, the ability of MRI to detect multifocal (within the same quadrant of the breast) and multicentric (within different quadrants) disease that was previously unsuspected ( Fig. 1 ) facilitates accurate staging. Incidental synchronous contralateral carcinomas have also been detected when screening the contralateral breast in patients with known cancer and may be the most compelling reason for performing breast MRI in the preoperative setting ( Fig. 2 ). In the patient with positive margins following an initial attempt at breast conservation (where MRI was not performed preoperatively), MRI can detect residual disease ( Fig. 3 ); and in the patient with inoperable locally advanced breast cancer, MRI may provide information to assess response to neoadjuvant chemotherapy ( Fig. 4 ). Suspected recurrence can be confirmed with MRI in the previously treated breast ( Fig. 5 ) and breast MRI is absolutely indicated in the patient with axillary node metastases with unknown primary ( Fig. 6 ). The final important indication is the use of MRI for screening in certain high-risk patients ( Fig. 7 ), which will be discussed elsewhere in this book.







Recent guidelines and recommendations
During the past few decades, as breast MRI has been incorporated into the clinical evaluation of the breast, it became apparent that standardization of image acquisition and terminology is extremely important. The American College of Radiology (ACR) Committee on Standards and Guidelines has published a document for the indications and performance of breast MRI in 2004. Recently, the Breast Imaging Reporting and Data System (BI-RADS) lexicon has added a section regarding breast MRI that has already been revised and further revisions are in progress. These efforts have been important in establishing the standards of reporting and the standards for patient selection. The existence of standardized guidelines in image acquisition and interpretation has helped disseminate this technology from academic centers into the community. Furthermore, the ACR is supporting efforts to establish a voluntary accreditation process for performing breast MRI. This will further standardize the acquisition of the MRI examination and provide high-quality imaging to women at those centers that are accredited. The revised recommendations for high-risk screening from the American Cancer Society (ACS) will no doubt accelerate this important accreditation process.
The ACS recently made a modification in the recommended screening guidelines to recommend annual screening MRI examination for certain high-risk women, which will be discussed briefly in this chapter.
Sensitivity and specificity issues in breast MR imaging
Breast MRI for cancer detection relies almost exclusively on the neovascularity associated with invasive carcinomas. The administration of an intravenous contrast agent such as gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) allows these lesions to be well visualized, particularly if subtraction imaging or chemical fat suppression sequences are used. Leaky capillaries and arterio-venous shunts allow contrast agents to leave the lesion rapidly over time resulting in the characteristic washout time intensity curves that can be seen with most but not all malignancies. Detection of invasive breast carcinoma is extremely reliable on MR imaging as the sensitivity approaches 100%. As the sensitivity for cancer detection is high, the negative predictive value of breast MRI is high. If no enhancement is present in the breast, and any possible technical mishap such as intravenous contrast extravasation has been excluded, there is an extremely high likelihood that no invasive carcinoma is present. Specificity is lower than sensitivity and therefore false positives can pose a problem in interpretation. False positives can be caused by high-risk lesions such as lobular carcinoma in situ (LCIS), atypical ductal hyperplasia (ADH), and atypical lobular hyperplasia (ALH), as well as benign masses such as fibroadenomas, papillomas, and lymph nodes. Additionally, fibrocystic changes, sclerosing adenosis, duct hyperplasia, and fibrosis can result in a benign biopsy. With experience, many of these lesions can be diagnosed as benign; however, false positives will always be an issue on MRI as they are on mammography and sonography.
Despite the reputation of high detection of cancer, false negative examinations with MRI do exist. It should be noted that false negatives have been reported with some well-differentiated invasive ductal carcinomas as well as invasive lobular carcinoma. Moreover, not all DCIS is detected on MRI. Although the sensitivity is very high for invasive carcinoma, the sensitivity DCIS has been reported in prior literature to be somewhat lower, possibly secondary to more variable angiogenesis associated with DCIS lesions and the variable appearance. But, more recent evidence suggests that the sensitivity for DCIS detection may actually be higher than previously reported now that high-resolution scanning techniques are more available and widely used and the patterns of DCIS on MRI are more recognized. Morphology may be more important than kinetics in the evaluation of DCIS; thus, a slightly modified interpretation approach is taken when evaluating for DCIS. Although more work needs to be performed in the MR assessment of in-situ disease, MRI does not currently have as high a negative predictive value for DCIS as with invasive cancer. Therefore, MRI is not able to exclude DCIS with current technology and cannot be used to exclude the need for biopsy of suspicious calcifications. Nevertheless, MRI can detect mammographically occult DCIS and is able to play a valuable role possibly in the preoperative assessment of DCIS, where extent of disease may be underestimated by mammography.
Because of the potential issue of a false negative examination, a negative MRI examination should not deter biopsy of a suspicious lesion (BIRADS 4 or 5) on mammography or ultrasound. Mammographically suspicious findings, such as suspicious calcifications, spiculated masses, or areas of distortion warrant appropriate biopsy, regardless of a negative MR examination. The MRI should ideally be interpreted in conjunction with all other pertinent imaging studies such as mammograms and ultrasounds to arrive at the best treatment option for the patient. With these limitations, breast MRI is best used as an adjunct test to conventional imaging, complementing but never replacing basic mammography and sonography.
Sensitivity and specificity issues in breast MR imaging
Breast MRI for cancer detection relies almost exclusively on the neovascularity associated with invasive carcinomas. The administration of an intravenous contrast agent such as gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) allows these lesions to be well visualized, particularly if subtraction imaging or chemical fat suppression sequences are used. Leaky capillaries and arterio-venous shunts allow contrast agents to leave the lesion rapidly over time resulting in the characteristic washout time intensity curves that can be seen with most but not all malignancies. Detection of invasive breast carcinoma is extremely reliable on MR imaging as the sensitivity approaches 100%. As the sensitivity for cancer detection is high, the negative predictive value of breast MRI is high. If no enhancement is present in the breast, and any possible technical mishap such as intravenous contrast extravasation has been excluded, there is an extremely high likelihood that no invasive carcinoma is present. Specificity is lower than sensitivity and therefore false positives can pose a problem in interpretation. False positives can be caused by high-risk lesions such as lobular carcinoma in situ (LCIS), atypical ductal hyperplasia (ADH), and atypical lobular hyperplasia (ALH), as well as benign masses such as fibroadenomas, papillomas, and lymph nodes. Additionally, fibrocystic changes, sclerosing adenosis, duct hyperplasia, and fibrosis can result in a benign biopsy. With experience, many of these lesions can be diagnosed as benign; however, false positives will always be an issue on MRI as they are on mammography and sonography.
Despite the reputation of high detection of cancer, false negative examinations with MRI do exist. It should be noted that false negatives have been reported with some well-differentiated invasive ductal carcinomas as well as invasive lobular carcinoma. Moreover, not all DCIS is detected on MRI. Although the sensitivity is very high for invasive carcinoma, the sensitivity DCIS has been reported in prior literature to be somewhat lower, possibly secondary to more variable angiogenesis associated with DCIS lesions and the variable appearance. But, more recent evidence suggests that the sensitivity for DCIS detection may actually be higher than previously reported now that high-resolution scanning techniques are more available and widely used and the patterns of DCIS on MRI are more recognized. Morphology may be more important than kinetics in the evaluation of DCIS; thus, a slightly modified interpretation approach is taken when evaluating for DCIS. Although more work needs to be performed in the MR assessment of in-situ disease, MRI does not currently have as high a negative predictive value for DCIS as with invasive cancer. Therefore, MRI is not able to exclude DCIS with current technology and cannot be used to exclude the need for biopsy of suspicious calcifications. Nevertheless, MRI can detect mammographically occult DCIS and is able to play a valuable role possibly in the preoperative assessment of DCIS, where extent of disease may be underestimated by mammography.
Because of the potential issue of a false negative examination, a negative MRI examination should not deter biopsy of a suspicious lesion (BIRADS 4 or 5) on mammography or ultrasound. Mammographically suspicious findings, such as suspicious calcifications, spiculated masses, or areas of distortion warrant appropriate biopsy, regardless of a negative MR examination. The MRI should ideally be interpreted in conjunction with all other pertinent imaging studies such as mammograms and ultrasounds to arrive at the best treatment option for the patient. With these limitations, breast MRI is best used as an adjunct test to conventional imaging, complementing but never replacing basic mammography and sonography.
Preoperative staging
Background
Over the past 30 years, surgical treatment for breast cancer has evolved from total mastectomy in all cases to breast conservation therapy (BCT) in most cases. BCT has moved from being an experimental treatment to the mainstay of surgical therapy as observational studies and randomized controlled trials have demonstrated similar survival between the BCT and mastectomy groups. This has occurred with considerable refinement in patient selection, surgical technique with emphasis on clear margins, and postsurgical radiation and chemotherapy. To arrive at a point where BCT followed by radiation and chemotherapy has resulted in recurrence rates of approximately 10% or greater is remarkable given that is has been known all this while that residual disease is left in the breast. Elaborate treatment algorithms have evolved based on extent of disease evaluation based on clinical and mammographic examination. Surgeons and oncologists have accepted and become comfortable with the probability of recurrence in a significant but small number of patients. When these important studies were taking place, film screen mammography, which has significant limitations, was the standard of care. These days we now have better methods of evaluating tumor load in the breast with MRI, which is far superior to mammography. Controversy arises because the treatment of breast cancer is working well enough and clinicians who are vested in the treatment algorithm do not want to consider the extra information that MRI has to offer. Additionally, MRI has not undergone the rigorous evaluation and study design that these prior studies, many of them randomized controlled trials, have undergone. Certainly, the aim of incorporating breast MRI into the evaluation of cancer would not be to unnecessarily increase the mastectomy rate but rather triage those patients to the more appropriate therapy with full knowledge of the tumor load up front instead of having to guess.
If used carefully, breast MRI has the potential to decrease positive margin rates and recurrence rates in patients with newly diagnosed breast cancer who are candidates for BCT. Breast MRI may also help identify those patients in whom positive margins are likely to arise if surgery is undertaken and those patients who may best benefit from mastectomy as the first-line therapy.
Traditional preoperative planning for breast cancer involves clinical examination and mammogram. Assessment of lesion size, presence of multifocality or multicentricity, and involvement of adjacent structures such as pectoralis muscles and chest wall is therefore dependent on a modality that has limitations and is imperfect. It has been well documented that while mammography has benefits in overall screening of the average-risk population, it is less promising when certain individual groups of women are analyzed, such as women with dense breast tissue. Dense tissue is common, especially in younger women. It has been shown that 62% of women in their 30s, 56% of women in their 40s, 37% of women in their 50s, and 27% of women in their 60s had at least 50% density on mammography. Aside from the inherent increased risk of breast cancer associated with increased density, mammographic detection of cancer is decreased where up to 50% of cancer in some series can go undetected, particularly in young women who are at increased risk. This observation that breast density can hinder evaluation of additional disease is important in the realm of preoperative staging.
Breast MRI can give helpful information for staging on tumor size and presence or absence of multifocal or multicentric disease, as well as whether the chest wall or pectoralis muscle is invaded. It has been well documented that MR defines the anatomic extent of disease more accurately than mammography. Many studies have shown that MRI is able to detect additional foci of cancer in the breast that has been overlooked by our conventional techniques. Several investigators have shown that MRI is able to detect additional foci of disease ( Fig. 8 ) in up to one third of patients, possibly resulting in a treatment change. MRI can potentially provide valuable information for preoperative planning in the single-stage resection of breast cancer. By using breast MRI as a complementary test to the conventional imaging techniques, more precise information can be obtained about the extent of breast cancer, improving patient care. There is no evidence that the additional cancer found by MRI is any different or less significant that the cancer found by mammography, ultrasonography, or physical examination.
Contraindications to Breast Conservation Therapy
A joint committee of the American College of Surgeons, American College of Radiology, and College of American Pathologists published standards for breast conservation in1992, which have been routinely updated. Absolute contraindications include inability of the surgeon to obtain negative margins after a reasonable number of surgeries, first or second trimester pregnancy, inability to undergo radiation (prior chest radiation, lupus, scleroderma, and so forth), and clinical or mammographically detected multicentric cancer.
Multicentric cancer detected on mammography or clinical examination occurs in approximately 10% or less of cases of breast cancer. MRI is able to detect multicentricity in 13% to 37% of patients. As multicentricity on mammography is a contraindication to BCT, one could extrapolate that additional disease on MRI would obviate BCT. If one compares the type of tumor that is detected as multicentric disease on MRI, it is no different from that detected by mammography. By all measurable standards they appear to be the same. Therefore why would we put more emphasis on the additional cancer that was detected by one modality as opposed to the other? Because the treatment trials were performed with mammography.
Presumably some of this multicentric disease results in recurrence at a later date. Even if we could identify 5% to 10% of patients who harbor additional multicentric disease that could presumably cause a recurrence, this would justify the use of preoperative MRI.
Recurrence
It has been long known that all disease is not eradicated by surgery alone. The rationale for delivering whole breast radiation (or even partial breast radiation) is that the surgeon has removed the bulk of the disease but that radiation is needed to treat remaining subclinical disease. It has been shown from a study of 282 mastectomy specimens (performed for unifocal breast cancer, assessed clinically and mammographically) that the majority of breasts (63%) have additional sites of cancer that were undetected by clinical examination or mammography. Additional foci of cancer were found pathologically in 20% within 2 cm of the index cancer (multifocal disease) and in 43% more than 2 cm away from the index cancer (disease that may or may not require mastectomy). There were 7% who had additional foci of carcinoma more than 4 cm away from the index cancer, likely representing cancer within a separate breast quadrant (multicentric disease likely requiring mastectomy).
Breast radiation therapy developed to fill a need to treat residual disease and has become a mainstay of the treatment of breast conservation of most patients whether or not residual disease exists. It has been well documented that radiation reduces local recurrence. However, until the use of MRI, it has not been possible to reliably identify those patients who harbor additional multifocal or multicentric cancer and who may be at increased risk of recurrence. While there has been a large body of work showing that MRI can document this additional disease there is a relative paucity of data to document that the recurrence rates are decreased with the addition of MRI. There is only one published paper that has addressed the impact of preoperative MRI on recurrence rates. It demonstrates that if MRI is performed, recurrence rates are lower. Unfortunately the study is flawed in that both groups of women (those that had MRI and those that did not) are not identical. Additionally, the tumor stage and types were different in the two groups. More studies examining this important issue need to be performed.
Positive Surgical Margins
One of the most important factors associated with local recurrence after lumpectomy is the status of the surgical margin. Standard surgical practice is to obtain clear margins even if this requires a second or sometimes third surgical procedure. It is assumed that reexcision to achieve clear margins is as effective as complete tumor removal in a single procedure.
In a significant percentage of patients, the surgeon may not get all the cancer at surgery. The imaging lesion on mammography or ultrasonography or the palpable lesion may be removed but the pathologic margins come back as close or positive. In these cases the surgeon usually recommends reexcision unless there is advanced age/comorbidities. The purpose of the return trip to the operating room is to further excise (usually blindly unless the postoperative mammogram shows residual calcifications) more tissue to hopefully get negative margins at the second surgery.
Positive margins increase the health care costs, as a return trip to the operating room is required as well as increasing anxiety and concern in the patient. Unless residual calcifications are identified, reexcision is usually performed blindly. Too little or too much tissue may be removed when there is no information about how much residual disease exists. Positive margin rates have been reported as high as 70%, although more conservative estimates report 30% to 50% of women undergoing breast conservation therapy may require additional surgery for positive margins. There is a real potential for MRI to aid the surgeon in mapping the amount of residual disease.
Controversies in Using Breast MR Imaging in Cancer Staging
Controversy exists regarding the use of MRI to stage breast cancer. Because breast cancer treatment has been successfully refined over the past decades there is appropriate concern about addition of MRI to the preoperative work-up of the known cancer. The general argument is that with breast conservation surgery followed by radiation therapy, recurrence rates are low: reported recurrence rates at 10 years are conservatively 10%. Early local recurrence is thought to be adversely related to patient outcome (likely reflecting residual disease not treated by surgery and radiation); however, recurrence that happens after 10 years is thought to probably not adversely affect patient outcome (likely a new primary).
In a study from Fox Chase Cancer Center looking at Stage I and Stage II breast cancer treated with breast conserving therapy, axillary node dissection, and radiation therapy, the reexcision rate was 59%. Final margin status was negative in 77%, positive in 12%, and close (2 mm or less) in 11%. Recurrence rates at 5 years were not significantly different (negative 4%, positive 5%, close 7%); however, at 10 years there was a significant difference in recurrence rates (negative 7%, positive 13%, close 21%). First of all, these results underline the high proportion of patients undergoing a second surgery for margins that were not clear. Second, the importance of obtaining final negative margins is directly related to a low recurrence rate. Additionally, the results demonstrate that even from a specialty cancer center, recurrence rates can be significant.
Another controversial area in the performance of preoperative MRI has been the argument that MRI results in too many mastectomies and too many false positive biopsies. While it is true that before the advent of percutaneous MRI biopsy there were too many patients having mastectomies for MRI lesions that were not evaluated before surgery and later proved to be benign, the same cannot be said today. If one examines the literature of how preoperative MRI changed the surgical approach, MRI converts a patient to mastectomy approximately 15% of the time. So it is the minority of patients undergoing preoperative evaluation who are converted to mastectomy. If you compare the number of MRI-prompted mastectomies with the number of women who have a recurrence at 10 years, they are surprisingly similar numbers. While there is no current evidence to directly suggest that MRI is detecting the disease that goes undetected, untreated, and ultimately results in a recurrence, it is an interesting question that warrants further examination. It is very likely in many cases that MRI is identifying disease that would likely cause a recurrence. Carefully designed studies are needed to evaluate this important question.
One recent study from Northwestern University in Chicago, Illinois, evaluated the impact of breast MRI on the surgical management of 155 women with newly diagnosed breast cancer. MRI identified 124 additional lesions in 73 patients. Change in surgical management occurred in 36 (23%) of 155. Lumpectomy was converted to mastectomy in 10 (6%) of 155. In 8 (80%) of 10 this was beneficial to the patient. In 2 (20%) 10 borderline lesions for BCT were converted to mastectomy on the basis of MRI where MRI overestimated disease. Overall, MRI resulted in a beneficial change in surgical management in 10% of newly diagnosed breast cancers. In these authors’ estimation, the detection of additional ipsilateral and contralateral cancers justifies the role of preoperative breast MRI. They also found the specificity improved during the course of the study with refinements in MR technique and increase in radiologist expertise. In their estimation, 10 women must undergo breast MRI to result in a benefit to 1 patient. They compare this to the prophylactic mastectomy data where six women must undergo bilateral mastectomy to benefit one woman. Therefore, as women with newly diagnosed breast cancer are high risk, this seems like a reasonable number. They also note that additional cancer identified on MRI regardless of size should be considered important. If surgeons believe it is important to clear lumpectomy margins of microscopic disease with further surgery to minimize the risk of local recurrence, it would follow that small foci detected on MRI should be considered important and also warrant excision.
Issues to Consider in Relation to Staging Controversy
Recurrence rates and positive margin rates vary throughout the country between practices and individual surgeons. Recurrence rates are cited as being low and decreasing, although no standardization or auditing occurs in most surgical practices. Positive margin rates necessitating return to the operating room for further reexcision are also not audited or standardized. Unlike breast imaging where benchmarks are published and auditing of a practice is routine, breast surgical practice is not as scrutinized or regulated. The impact of breast MRI on an individual surgical practice will depend on the positive margin rate as well as the recurrence rate of that individual practice.
From the radiology perspective, these issues raise many questions, such as what lesion size can we safely ignore on MRI. If we are committed to using radiation on all patients, perhaps MRI is too sensitive in detecting cancer in general. For our current treatment algorithms that involve the use of radiation, MRI is likely detecting subclinical disease that radiation therapy would adequately treat. In general, it is recognized that disease 1 cm or less will be treated with radiation. On the other hand, MRI may detect additional disease that would not be treated with adjuvant therapy, particularly invasive cancers that are 1 cm or greater. If we desire to continue in the treatment algorithms that have evolved and that use postoperative radiation therapy, the challenge is identifying what is and what is not significant disease. At this time, identification of significant disease that will not be treated with radiation therapy is not possible and all additional disease is treated surgically. Performing breast MRI to possibly prevent recurrence may have benefit to a significant number of breast conservation patients, namely those who will recur (at least 10% by 10 years). Certainly it would be better to prevent recurrence with the attendant health care and personal costs if one could. Trials that involve radiologists as well as radiation oncologists and surgeons are needed to get information to potentially identify these patients so that optimum care is delivered to our patients.
Examining the Contralateral Breast in the Staging MR Imaging Examination
Probably the most compelling reason to perform breast MRI in the patient with known cancer is the assessment of the contralateral breast. It has been well documented that MRI is able to detect occult contralateral breast cancer in approximately 4% to 6% of patients. These cancers are sometimes the more significant lesion and may alter the staging of the patient. Furthermore, knowledge of the extent of disease in both breasts allows optimal treatment options to be discussed at the outset with the patient instead of many years later when the patient develops her contralateral primary. To ignore the opposite breast and assume that the adjuvant chemotherapy will treat unsuspected contralateral disease does not make clinical sense when we expend so much energy, time, and resources to treat the known cancer.
Who should Undergo Preoperative Breast MR Imaging?
All patients with a new diagnosis of breast cancer should arguably undergo bilateral MRI examination preoperatively. There are several reasons for this statement. First, the high rate of contralateral carcinoma justifies the use of routine bilateral MRI. Also, for those patients with true multicentric disease the appropriate therapy can be done up front. A conservative recurrence rate of 10% at 10 years certainly justifies the use of a single MRI examination at the time of treatment planning to identify those patients who may benefit from mastectomy. Last, the index lesion is better defined on MRI so that the surgeon may have a better chance at obtaining negative margins at the first attempt of conservation. One study from Stanford demonstrates that bracketing of the lesion by MRI may facilitate complete removal of the lesion if a large DCIS component is not present. Therefore, performing preoperative MRI may increase the chance that the surgeon obtains a negative margin at the initial surgery. More data are needed to address this potential use of MRI to decrease the positive margin rate.
At the very least, perhaps the best patients for preoperative MRI are those who are known to have high rates of positive margins and recurrence. For example, young patients, all patients with dense or moderately dense breasts, and patients with difficult tumor histology such as infiltrating lobular carcinoma, DCIS, and tumors with extensive intraductal component (EIC), where tumor size assessment is difficult on mammography or ultrasound ( Figs. 9 and 10 ). EIC is when the invasive carcinoma has an associated greater than 25% component of DCIS. EIC is associated with positive margins and high recurrence rates. Interestingly, as MRI is more sensitive to DCIS detection than mammography, it may become the test of choice to evaluate patients preoperatively. Several trials are under way to assess this potential use of MRI.