MR imaging-guided interventions are well established in routine patient care in many parts of the world. There are many approaches, depending on magnet design and clinical need, based on MR imaging providing excellent inherent tissue contrast without ionizing radiation risk for patients. MR imaging-guided minimally invasive therapeutic procedures have advantages over conventional surgical procedures. In the genitourinary tract, MR imaging guidance has a role in tumor detection, localization, and staging and can provide accurate image guidance for minimally invasive procedures. The advent of molecular and metabolic imaging and use of higher strength magnets likely will improve diagnostic accuracy and allow targeted therapy to maximize disease control and minimize side effects.
MR imaging–guided interventions are a well-established form of routine patient care in many centers around the world. There are many different approaches, depending on magnet design and clinical need. The rationale behind this is based initially on MR imaging providing excellent inherent tissue contrast, without ionizing radiation risk for patients. MR imaging–guided minimally invasive therapeutic procedures have major advantages over conventional surgical procedures. In the genitourinary tract, MR imaging guidance can play a role in tumor detection, localization, and staging and can provide accurate image guidance for minimally invasive procedures for the confirmation of pathology, tumor treatment, and treatment monitoring. Depending on the body part accessed, a customizable magnet bore configuration and magnetic resonance (MR)-compatible devices can be made available. The advent of molecular and metabolic imaging and the use of higher strength magnets likely will improve diagnostic accuracy and allow patient-specific targeted therapy, designed to maximize disease control and minimize side effects.
Genital tract: female
One of the most unique and exciting MR-guided interventional procedures in the female pelvis is MR-guided focused ultrasound surgery (MRgFUS). In addition, MR is used to guide other interventions and therapies, such as biopsies and gynecologic tumor treatments. The latter have been done in several centers, guiding the placement of radiation catheters for delivery of high-dose radiation in cervical or endometrial cancer.
Magnetic resonance–guided focused ultrasound surgery for treating uterine fibroids
Uterine fibroids are the most common female pelvic tumor, occurring in approximately 25% of women. Although many patients remain asymptomatic, others suffer from symptoms, such as pelvic pain, menorrhagia, dysmenorrhagia, dyspareunia, urinary frequency, and infertility. Ultrasound (US) usually is the first diagnostic imaging modality of choice for fibroids, demonstrating a well-defined, usually hypoechoic mass. Providing good inherent tissue contrast, MR imaging is the optimal modality for fibroid detection, accurate localization, and volumetrics.
A wide spectrum of treatment options for uterine fibroids exists, ranging from expectant waiting to medical management to myomectomy to hysterectomy. Women, however, increasingly are seeking less invasive treatment options, perhaps motivated by fertility preservation and the possibility of reduced postprocedure recovery time. A good example of a less invasive choice is uterine artery embolization, a procedure that has demonstrated significant growth and interest since its introduction in 1995. Only MRgFUS, however, is completely noninvasive. Approved by the United States Food and Drug Administration (FDA) in October 2004, much of the worldwide experience with MRgFUS has been with treatment of uterine fibroids, with more than 3500 patients treated to date.
Fundamentals of Magnetic Resonance–Guided Focused Ultrasound Surgery
The potential surgical application of focused ultrasound surgery (FUS) was first demonstrated in 1942. Since then, it has been evaluated extensively in animal and human brains and in the kidney, prostate, liver, bladder, and eye within clinical trials. Clinical acceptance, however, was hampered because of the difficulty in controlling the focal spot position, defining the beam target precisely, and coping with the lack of feedback about thermal damage.
MR imaging can satisfy the requirements of FUS, having excellent anatomic resolution and high sensitivity for tumor visualization, thereby offering accurate planning of the tissue to be targeted. By exploiting the temperature dependence of the water proton resonant frequency, MR-based temperature mapping is possible. This allows for targeting of the beam during subthreshold US exposures and online estimation of the ablated volume. Phase imaging is used to estimate the temperature-dependent proton resonant-frequency shift using a fast spoiled gradient-recalled-echo sequence (SPGR). Therefore, obtaining temperature-sensitive MR images before, during, and after each sonication can monitor tissue temperature elevations, including any slight elevations in normal adjacent surrounding tissue, thereby preventing damage.
Magnetic Resonance–Guided Focused Ultrasound Surgery Equipment for Fibroid Treatment
Sonications are performed using an MR-compatible focused US system that is built into a table that docks with a compatible MR scanner. The system consists of a focused piezoelectric phased-array transducer (208 elements, frequency 0.96–1.14 MHz) that is located within the specially designed table surrounded by a water tank. A thin plastic membrane covers the water tank and allows the US beam to propagate into the tissue. Patients lie in a prone position in the magnet, with the anterior abdominal wall positioned over the water tank. The location of the focal spot is controlled electronically by the transducer array that controls the volume of coagulation necrosis.
Patient Selection for Magnetic Resonance–Guided Focused Ultrasound Surgery of Uterine Fibroids
The FDA has approved this procedure for premenopausal women who have symptomatic uterine fibroids and who have no desire for future pregnancy. This treatment is not indicated for pregnant women, postmenopausal women, or those who have contraindications to contrast-enhanced MR imaging. If multiple fibroids are present, clinical symptomatology and accessibility to the target fibroids are reviewed and a target fibroid is selected. The anterior abdominal wall is evaluated for extensive scarring. Those women who have such scarring are excluded from treatment because of the risk for skin burns.
Treatment Planning
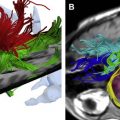
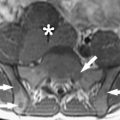
Immediately before treatment, T2-weighted fast spin-echo images in three orthogonal planes are obtained to plan the beam path to the targeted lesion. The MR images are analyzed to evaluate the area to be treated for possible obstructions. Although patients who have extensive anterior abdominal wall scarring in the beam path generally are excluded at screening, it may be possible, however, to treat women who have abdominal wall scarring that is not extensive by angling the beam path, ensuring that the scar is not traversed ( Fig. 1 ). Filling of the urinary bladder by Foley catheter clamping also may help in moving the uterus and selected fibroid into a position away from the abdominal wall scar. Coursing bowel loops lying anterior to the uterus at the level of the uterine fibroid also may cause treatment-planning difficulties. Placement of a gel spacing device may allow the bowel loops to be displaced out of the treatment field, thereby enlarging the acoustic window and allowing for greater treatment volume ( Fig. 2 ).
Clinical Trials in the Treatment of Uterine Fibroids with Magnetic Resonance–Guided Focused Ultrasound Surgery
Multicenter clinical trials investigating the use of MRgFUS in the treatment of uterine fibroids, which subsequently resulted in device labeling by the FDA, were performed at five medical centers across the United States in addition to centers in the United Kingdom, Germany, and Israel. Follow-up of many patients is ongoing.
Enrollment for phase I/II began in 1999 to assess the safety and feasibility of MRgFUS in the treatment of fibroids. Eligible patients underwent MRgFUS followed by hysterectomy, and subsequent pathologic examination of the uterus and fibroid showed that MRgFUS did result in hemorrhagic necrosis in the area of nonperfusion on the post-treatment MR.
Phase III of the clinical trial involved treatment of larger volumes of fibroids in women who had symptomatic uterine fibroids who otherwise would have opted for hysterectomy ( Fig. 3 ). To date, the longest-term follow-up—in 359 patients—is up to 24 months. These patients reported durable symptom relief. Those who had a greater nonperfused treatment volume fared better, with fewer of these patients undergoing additional fibroid treatment. These findings concur with those of Fennessy and colleagues, where greater clinical outcome was found in those treated with a modified treatment protocol that allowed for greater nonperfused fibroid volumes post treatment.
Magnetic resonance–guided focused ultrasound surgery for treating uterine fibroids
Uterine fibroids are the most common female pelvic tumor, occurring in approximately 25% of women. Although many patients remain asymptomatic, others suffer from symptoms, such as pelvic pain, menorrhagia, dysmenorrhagia, dyspareunia, urinary frequency, and infertility. Ultrasound (US) usually is the first diagnostic imaging modality of choice for fibroids, demonstrating a well-defined, usually hypoechoic mass. Providing good inherent tissue contrast, MR imaging is the optimal modality for fibroid detection, accurate localization, and volumetrics.
A wide spectrum of treatment options for uterine fibroids exists, ranging from expectant waiting to medical management to myomectomy to hysterectomy. Women, however, increasingly are seeking less invasive treatment options, perhaps motivated by fertility preservation and the possibility of reduced postprocedure recovery time. A good example of a less invasive choice is uterine artery embolization, a procedure that has demonstrated significant growth and interest since its introduction in 1995. Only MRgFUS, however, is completely noninvasive. Approved by the United States Food and Drug Administration (FDA) in October 2004, much of the worldwide experience with MRgFUS has been with treatment of uterine fibroids, with more than 3500 patients treated to date.
Fundamentals of Magnetic Resonance–Guided Focused Ultrasound Surgery
The potential surgical application of focused ultrasound surgery (FUS) was first demonstrated in 1942. Since then, it has been evaluated extensively in animal and human brains and in the kidney, prostate, liver, bladder, and eye within clinical trials. Clinical acceptance, however, was hampered because of the difficulty in controlling the focal spot position, defining the beam target precisely, and coping with the lack of feedback about thermal damage.
MR imaging can satisfy the requirements of FUS, having excellent anatomic resolution and high sensitivity for tumor visualization, thereby offering accurate planning of the tissue to be targeted. By exploiting the temperature dependence of the water proton resonant frequency, MR-based temperature mapping is possible. This allows for targeting of the beam during subthreshold US exposures and online estimation of the ablated volume. Phase imaging is used to estimate the temperature-dependent proton resonant-frequency shift using a fast spoiled gradient-recalled-echo sequence (SPGR). Therefore, obtaining temperature-sensitive MR images before, during, and after each sonication can monitor tissue temperature elevations, including any slight elevations in normal adjacent surrounding tissue, thereby preventing damage.
Magnetic Resonance–Guided Focused Ultrasound Surgery Equipment for Fibroid Treatment
Sonications are performed using an MR-compatible focused US system that is built into a table that docks with a compatible MR scanner. The system consists of a focused piezoelectric phased-array transducer (208 elements, frequency 0.96–1.14 MHz) that is located within the specially designed table surrounded by a water tank. A thin plastic membrane covers the water tank and allows the US beam to propagate into the tissue. Patients lie in a prone position in the magnet, with the anterior abdominal wall positioned over the water tank. The location of the focal spot is controlled electronically by the transducer array that controls the volume of coagulation necrosis.
Patient Selection for Magnetic Resonance–Guided Focused Ultrasound Surgery of Uterine Fibroids
The FDA has approved this procedure for premenopausal women who have symptomatic uterine fibroids and who have no desire for future pregnancy. This treatment is not indicated for pregnant women, postmenopausal women, or those who have contraindications to contrast-enhanced MR imaging. If multiple fibroids are present, clinical symptomatology and accessibility to the target fibroids are reviewed and a target fibroid is selected. The anterior abdominal wall is evaluated for extensive scarring. Those women who have such scarring are excluded from treatment because of the risk for skin burns.
Treatment Planning
Immediately before treatment, T2-weighted fast spin-echo images in three orthogonal planes are obtained to plan the beam path to the targeted lesion. The MR images are analyzed to evaluate the area to be treated for possible obstructions. Although patients who have extensive anterior abdominal wall scarring in the beam path generally are excluded at screening, it may be possible, however, to treat women who have abdominal wall scarring that is not extensive by angling the beam path, ensuring that the scar is not traversed ( Fig. 1 ). Filling of the urinary bladder by Foley catheter clamping also may help in moving the uterus and selected fibroid into a position away from the abdominal wall scar. Coursing bowel loops lying anterior to the uterus at the level of the uterine fibroid also may cause treatment-planning difficulties. Placement of a gel spacing device may allow the bowel loops to be displaced out of the treatment field, thereby enlarging the acoustic window and allowing for greater treatment volume ( Fig. 2 ).
Clinical Trials in the Treatment of Uterine Fibroids with Magnetic Resonance–Guided Focused Ultrasound Surgery
Multicenter clinical trials investigating the use of MRgFUS in the treatment of uterine fibroids, which subsequently resulted in device labeling by the FDA, were performed at five medical centers across the United States in addition to centers in the United Kingdom, Germany, and Israel. Follow-up of many patients is ongoing.
Enrollment for phase I/II began in 1999 to assess the safety and feasibility of MRgFUS in the treatment of fibroids. Eligible patients underwent MRgFUS followed by hysterectomy, and subsequent pathologic examination of the uterus and fibroid showed that MRgFUS did result in hemorrhagic necrosis in the area of nonperfusion on the post-treatment MR.
Phase III of the clinical trial involved treatment of larger volumes of fibroids in women who had symptomatic uterine fibroids who otherwise would have opted for hysterectomy ( Fig. 3 ). To date, the longest-term follow-up—in 359 patients—is up to 24 months. These patients reported durable symptom relief. Those who had a greater nonperfused treatment volume fared better, with fewer of these patients undergoing additional fibroid treatment. These findings concur with those of Fennessy and colleagues, where greater clinical outcome was found in those treated with a modified treatment protocol that allowed for greater nonperfused fibroid volumes post treatment.
Cryotherapy for uterine fibroids
MR-guided cryotherapy is a minimally invasive procedure. It involves a percutaneous approach in an interventional setting with multiple (1 to 5) needle-like 17-G cryotherapy probes. Each probe creates a tear-drop shaped volume of frozen tissue about its tip (approximately 2.5-mm diameter); the simultaneous use of multiple probes gives a larger volume of treated tissue in the same time frame as treating with a single probe. The freeze is provided by pressurized argon gas that circulates within the probe. Typical treatments involve a cycling of the gas that delivers a freeze-thaw-freeze to destroy tissue, with each stage of the cycle 10 to 15 minutes in duration. MR imaging–guided cryotherapy has been evolving through experiment and clinical use during the past 20 years to target a range of tumors in various organ systems. Compared with US that has shadow artifacts, visibility of the ice ball for monitoring is not as limited.
There are several promising reports of MR imaging–guided cryotherapy to treat symptomatic patients who have uterine fibroids. During cryotherapy, the ice appeared as a signal void in the image as a result of the short MR relaxation time of the solid ice, giving a clear demarcation between frozen and unfrozen tissue. Though all reported relief from deletorious symptoms, short-term clinical outcome, however, is reported in only 8 of 9 treated patients, who demonstrated on average, 65% volume reduction in uterine size.
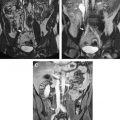
One of these studies was performed transvaginally, with the investigators proposing that such an approach had the advantage of providing direct access, especially for submucosal tumors. Procedures usually are performed with epidural anesthesia in a horizontally open MR imaging scanner with multiple 2- to 3-mm cryotherapy probes ( Fig. 4 ). Gradient-echo and T2-weighted spin-echo sequences were used to guide probe placement and monitor the treatment cycle of freeze-thaw-freeze ( Fig. 5 ).
Percutaneous ablation of fibroids is a nascent procedure and not practiced widely. This method of ablation has found a place in treating other parts of the body but not necessarily treating uterine fibroids, possibly because of the recent emergence of other minimally invasive procedures, such as uterine artery embolization, or noninvasive procedures, such as MRgFUS.
Genital tract: male
The leading cause of cancer death in men over 50, prostate cancer, affects one man in six in his lifetime. The American Cancer Society estimates that in 2007 in the United States, 218,890 new cases of prostate cancer will be diagnosed, and approximately 27,050 men will die of the disease. There is only a 33% 5-year survival rate in men who have metastatic disease, making early tumor detection and localized treatment a necessity.
MR imaging–guided prostate biopsy
Early diagnosis and cancer localization within the prostate gland usually are found through digital examination and serum prostate-specific antigen (PSA) measurement, followed by transrectal ultrasound (TRUS)-guided biopsy. Image-guided prostate biopsy with ultrasound (US) has become a universally accepted tool, but because of a low sensitivity and specificity for tumor detection, interest continues in the development of a more accurate technique. In addition, for men who have increasing PSA levels and repeatedly negative TRUS-guided prostate biopsies (the concern being that a sampling error may result in a false-negative biopsy), for those in whom a transrectal biopsy is not possible, or for those who are reluctant to undergo transrectal biopsy because of its recognized complications, such as infection, hematuria, hematospermia, and rectal bleeding, an alternative approach may be necessary.
MR imaging can outline prostate architecture and substructure. Although the specificity for diagnosis may be limited, MR imaging can demonstrate suspicious nodules in the peripheral zone, the most common site for prostate cancer. On T2-weighted images, tumor is demonstrated most commonly by focal or diffuse regions of decreased signal intensity relative to the high-signal-intensity normal peripheral zone. MR imaging is used most routinely for staging men who have known cancer. The reported accuracy of prostate cancer detection and staging on MR images varies widely, with reports of accuracy ranging from 54% to 93%, likely because of differences in techniques and interobserver variability.
Its role in detection and characterization, particularly in the initial diagnosis of high-risk patients or those who have previous negative biopsy findings but persistently high PSA levels, is increasing as techniques such as MR spectroscopy imaging (MRSI) and dynamic contrast enhancement become more widely available. The ultimate role and application in clinical practice, however, remain controversial. MR imaging contributes significant incremental value to TRUS-guided biopsy and digital rectal examination in cancer detection and localization in the prostate. It offers an excellent second-line alternative to those who have failed to obtain a diagnosis with conventional methods.
MR Imaging–Guided Prostate Biopsy: Technique
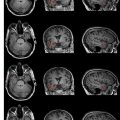
Two basic strategies have been explored for MR imaging–guided prostate biopsy. The first is coregistration of previously acquired diagnostic MR images to TRUS images, localizing suspected tumor lesions on MR and correlating these locations to the US. The second strategy is stereotactic needle interventions within diagnostic MR scanners using careful patient positioning. By implementing surgical navigation software originally developed for neurosurgery and adapting the technical capabilities of MR imaging–guided prostate brachytherapy in an open configuration magnet, biopsy of suspected tumor foci in the peripheral zone is made possible ( Fig. 6 ). In addition, the feasibility of transrectal needle access to prostate tumors has been assessed in a closed-bore 1.5-T magnet in a small number of patients, which potentially could provide for additional functional and spectroscopic imaging in comparison with a 0.5-T scanner. The procedure requires the use of a specialized device that consists of a needle guide and support system. The same guidance also has been used recently in a 3-T system. Larger studies of clinical usefulness, however, are necessary. With progress in biologic imaging of the prostate gland, it is likely that MR imaging guidance will play an increasing role in the diagnosis and treatment of prostate cancer.
MR Imaging–Guided Prostate Biopsy: The Future
The move toward targeted interventions, for diagnosis and treatment, underscores the need for precise image-guided needle placement. Based on a patient’s anatomy and lesion detection in pretreatment MR imaging, a graphic planning interface that allows desired needle trajectories to be specified, through MR-compatible robotic assistance, recently has been described. Avoiding the limitations of a fixed-needle template is a positive move forward for tissue sampling and treatment. As the field of prostate imaging moves to higher strength magnets, namely 3 T, the biopsy devices are reconfigured to allow sampling in a closed-bore environment. A recent study of prostate biopsy using 3-T MR imaging guidance described it as a promising tool for detecting and sampling cancerous regions in patients who have known prostate cancer ; however, the role (even at 3 T) of MR imaging–guided prostate biopsy as a screening tool in patients who have elevated PSA levels and recent previous negative biopsy remains to be determined.
MR imaging–guided brachytherapy for prostate cancer
Established options for the management of localized prostate cancer include one or a combination of the following: radical prostatectomy, external beam radiation therapy, brachytherapy, or watchful waiting. In radiation therapy, the goal is to achieve the prescribed dose throughout the prostate gland while minimizing toxicity to adjacent structures and minimizing morbidity from the procedure. Prostate brachytherapy is one of the more popular radiation methods in the prostate and involves the percutaneous placement of I-125 radiation seeds into the gland under image guidance. This is done most commonly with TRUS. It also can be done with MR guidance and the goal in both procedures is to optimize seed placement and allow maximal dose to the prostate peripheral zone tumor and minimal dose to the urethra and rectum.
Imaging-guided radiation therapy, therefore, allows directed tumor treatment, decreasing the chances of disease spreading outside the gland, while healthy prostate tissue and its neighboring structures are not overdosed. This is extremely important for structures such as the urethra, in which over-radiation may cause stricture and fistualization that can be avoided with good image guidance. Radiation dose fall-off is sharp at the rectal wall and at the urethra. Unlike external beam radiotherapy, there is no entrance or exit dose. Brachytherapy, therefore, has the potential to achieve superior tumor control with decreased morbidity and side effects. It is not, however, without its own set of complications, such as rectal irritation and ulceration, incontinence, and impotence resulting from inadvertent delivery of radiation dosing to the rectum, bladder, and urethra.
Patient Selection
Low-risk prostate cancer patients who have a high probability of organ-confined disease are screened appropriately with an endorectal coil MR for potential treatment with brachytherapy monotherapy. Most centers include patients who have stage T1-T2a (according to the American Joint Committee on Cancer/International Union Against Cancer 1997 staging), PSA level of 10 ng/mL or less, and a Gleason score of 6 or lower. The few contraindications to the procedure include prior transurethral resection of the prostate or morbid obesity (equipment cannot sustain the weight).
Procedure
MR-guided prostate brachytherapy using open configuration 0.5-T and 1.5-T scanners are described. Using the open 0.5-T magnet, patients are placed supine between the two magnets in the lithotomy position under general anesthesia. A Foley catheter is inserted, the skin is prepared and draped in a sterile fashion, and a template for needle guidance is placed against the perineum. A rectal obturator then is placed and T2-weighted images are acquired in the axial, coronal, and sagittal planes and used to outline the urethra, peripheral zone, and anterior rectal wall. Surgical simulation software outlines these areas, and the targeted volume is calculated using designated planning software. Seed number and depth of catheter insertion are calculated.
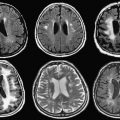
While gradient-echo MR images are obtained in real time, seed-loaded catheters then are positioned in the prostate gland ( Fig. 7 ). The images are compared to their intended locations, according to the radiation therapist plan. Dose-volume histograms of the urethra, anterior rectal wall, and target volume are obtained before final deployment of seeds. Six weeks after the procedure, CT imaging (to identify the seeds accurately) and MR imaging (for prostate anatomic correlation) are fused to calculate the final dose distribution to the gland and surrounding tissues.