Diaphragm
Mark C. Liszewski
Evan J. Zucker
Bernard F. Laya
Ricardo Restrepo
Edward Y. Lee
INTRODUCTION
The diaphragm is a large, flat, and dome-shaped musculotendinous structure that separates the thoracic and abdominal cavities, and is the primary muscle that drives respiration. The diaphragm consists of a peripheral muscular part and central tendinous part. It develops from four main components, which include the septum transversum, the pleuroperitoneal membrane, the medial dorsal portion of primary esophageal mesentery, and marginal ingrowths of the body wall. Due to its complex embryological development, the diaphragm is subject to a number of congenital anomalies. In addition, neoplasms and traumatic injuries can also result in diaphragmatic abnormalities in the pediatric population.
In this chapter, normal anatomy and variants of the diaphragm are discussed, up-to-date techniques for imaging the diaphragm are described, and congenital and acquired disorders that may affect the diaphragms of pediatric patients are reviewed.
ANATOMY
Embryology
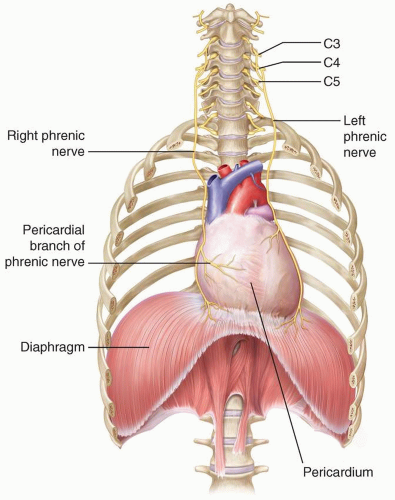
The diaphragm begins to develop during the 4th week of gestation, and by the 7th week, it has finished forming from fusion of the pleuroperitoneal membrane, the horizontal septum transversum, the mesentery of the esophagus, and the musculature of the lateral body wall1,2 (Fig. 6.1). The septum transversum is located in the central portion of the diaphragm and goes on to become a nonmuscular tendon in the dome called the central tendon. The portion of the diaphragm containing muscle fibers is largely derived from the pleuroperitoneal membranes, which become muscularized after myoblasts migrate to them from the cervical myotomes. The diaphragm receives blood from three main sources, including (1) branches of the internal thoracic arteries (i.e., the pericardiophrenic and musculophrenic arteries), (2) the superior phrenic arteries, and (3) the lower internal intercostal arteries. The innervation to the diaphragm is also cervical, supplied by the C3, C4, and C5 nerve roots (Fig. 6.2). A portion of the pleuroperitoneal membrane in the posterolateral portion of each diaphragm does not become muscularized and goes on to become the fibrous lumbocostal trigone.2 The remainder of the diaphragm develops from the structures of the lateral body wall at the periphery and the dorsal mesentery of the esophagus in the midline.2
Normal Development and Anatomy
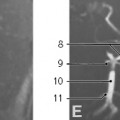
The fully developed diaphragm is composed of skeletal muscle and tendon with attachments to the sternum, ribs, and lumbar vertebrae. The posteromedial diaphragm is composed of thickened muscle bundles called crura, which attach to the L1, L2, and L3 vertebrae on the right and the L1 and L2 vertebrae on the left.1,3 The diaphragm separates the thoracic and abdominal cavities and has three openings that allow passage of structures between the chest and abdomen: the aortic, esophageal, and inferior vena caval hiatuses (Fig. 6.3). The aortic hiatus contains the aorta, thoracic duct, azygos vein, and hemiazygos vein.1 The esophageal hiatus contains the esophagus, vagus nerve, sympathetic nerves, and esophageal branches of the left gastric arteries and veins.1 The inferior vena caval hiatus contains the inferior vena cava and branches of the right phrenic nerve.1 The diaphragm is dome-shaped, and the inferior vena caval, esophageal, and aortic hiatuses are located at approximately the levels of T8-T9, T10, and T12, respectively.
Anatomic Variants
The normal anterior diaphragm has three possible appearances on cross-sectional imaging, depending on the position of the middle leaflet of the central tendon in relation to the xiphoid process.4 In a type I configuration, the middle leaflet is superior to the xiphoid process; in a type II configuration, the middle leaflet is located inferior to the xiphoid process; and in a type III configuration, the middle leaflet is located at
the same level as the xiphoid process.4 Type I occurs in 48%, type II occurs in 28%, and type III occurs in 11%.4
the same level as the xiphoid process.4 Type I occurs in 48%, type II occurs in 28%, and type III occurs in 11%.4
The normal diaphragmatic crura are relatively larger in children than in adults.5 This may cause the crura to have a nodular appearance, particularly in children under5 years of age5 (Fig. 6.4). Familiarity with this normal finding is important to avoid mistaking the normal crura of a child for adenopathy or other pathology.
IMAGING TECHNIQUES
The diaphragm may be imaged with a variety of different imaging modalities, and each modality has strengths and weaknesses. For example, modalities with real-time imaging capability such as fluoroscopy and ultrasound are superior for evaluation of diaphragmatic motion. Modalities with excellent contrast resolution, such as CT and MRI, are superior for evaluating intrinsic disorders of the diaphragm such as neoplasm. The approach to imaging disorders of the diaphragm depends largely on the disorder in question, and it is important to understand the capabilities of each modality.
Radiography
Chest radiographs are an excellent first-line imaging test for the evaluation of most disorders of the pediatric chest, including the diaphragm. Radiographs are relatively easy to perform, inexpensive, and associated with a low-radiation dose. Although fine anatomic detail of the diaphragm is not well depicted on chest radiographs, the majority of diaphragmatic disorders produce abnormal findings.
 FIGURE 6.3. The three major diaphragmatic hiatuses include the inferior vena caval hiatus, the esophageal hiatus, and the aortic hiatus. |
 FIGURE 6.4. A 1-year-old girl with a normal nodular appearance of the diaphragmatic crura. Axial enhanced CT image shows a normal nodular appearance of the left diaphragmatic crus (arrow). |
On chest radiography, the normal diaphragm appears as a dome-shaped structure separating the thoracic and abdominal cavities (Fig. 6.5A). The diaphragm can be visualized by radiography because it is bordered by air-filled lung superiorly; however, it is not seen as a discrete structure because it is silhouetted by the abdominal organs inferiorly. The position of the diaphragm on a chest radiograph changes with the degree of inspiration. Standard chest radiographs are obtained at end inspiration, and the dome of the right hemidiaphragm typically projects at the level of the anterior sixth rib, while the dome of the left projects at the anterior seventh rib.3,6 However, wide variability in the normal position of the diaphragm is worth noting.7 On lateral chest radiographs (Fig. 6.5B), the right hemidiaphragm can be seen in its entirety, while the left is silhouetted anteriorly by the heart.
Although chest radiographs do not depict the diaphragm in great detail, most disorders of the pediatric diaphragm result in abnormal findings on chest radiography. The radiograph, therefore, serves as an excellent screening tool. For example, chest radiographs in congenital diaphragmatic hernia (CDH) often depict dramatic findings of herniated abdominal structures in the chest in spite of often-poor visualization of the actual diaphragmatic defect. Diaphragmatic neoplasms may present on chest radiographs with pleural effusion and opacity adjacent to the lung base, but the origin of a mass may only be clear after obtaining further imaging with ultrasound, CT, or MRI. Diaphragmatic paralysis may result in elevation of the hemidiaphragm on a chest radiograph, and suspected abnormal diaphragmatic motion may be confirmed with a modality that has real-time imaging capability, such as fluoroscopy or ultrasound.
Fluoroscopy
Fluoroscopy produces serial radiographs that can be viewed in real time and replayed as a cine clip, allowing for assessment of moving structures like the diaphragm. A fluoroscopic assessment of the diaphragm may be prompted by abnormal findings on chest radiography or difficulty weaning from a ventilator. Depending on the patient’s ability to follow instructions, images may be obtained during quiet breathing, deep breathing, coughing, and/or sniffing. The normal diaphragm should demonstrate symmetric inferior motion during inspiration
and superior motion with expiration. Diaphragmatic paralysis causes absent or paradoxical movement of the affected portion of the diaphragm. Diaphragmatic eventration causes delayed movement of the diaphragm, although imaging findings are often indistinguishable from diaphragmatic paralysis.
and superior motion with expiration. Diaphragmatic paralysis causes absent or paradoxical movement of the affected portion of the diaphragm. Diaphragmatic eventration causes delayed movement of the diaphragm, although imaging findings are often indistinguishable from diaphragmatic paralysis.
Fluoroscopy utilizes x-rays to obtain images, and every effort should be made to limit patient radiation dose according to the ALARA (as low as reasonably achievable) principle. Strategies to limit potentially harmful x-ray exposure include removing the antiscatter grid in small patients, limiting electronic magnification, collimating to include only the area of interest, and considering alternative tests.8 Largely due to concerns about radiation exposure, ultrasound has replaced fluoroscopy as the first-line imaging test for evaluation of diaphragmatic motion in most scenarios, particularly in the pediatric population. However, fluoroscopy still has a role in selected cases where ultrasound is not diagnostic or unavailable.
Ultrasound
Due to portability and capability of imaging without ionizing radiation, ultrasound has become the preferred imaging modality for the evaluation of diaphragmatic motion in children. Ultrasound can also be useful in the evaluation of CDH,2,9 diaphragmatic eventration,10,11 traumatic rupture of the diaphragm,12,13 and diaphragmatic tumor.14,15
Technical aspects of imaging the diaphragm should be adjusted according to patient age and body habitus. Highfrequency transducers (7.5 to 15.0 MHz) produce high detail images but poor penetration of overlying subcutaneous fat and are, thus, not well suited to imaging deeper structures. Lower-frequency transducers (<5 MHz) produce less detailed images but are able to penetrate to greater depth. Therefore, high-frequency transducers are often more useful in infants and young children with less subcutaneous fat, while lower-frequency transducers are often more useful in older children who have more subcutaneous fat.16
Each hemidiaphragm can be imaged in transverse, sagittal, and oblique planes, appearing as a thin hypoechoic muscular layer adjacent to echogenic aerated lung (Fig. 6.6). M-mode imaging of each hemidiaphragm can be obtained in the sagittal plane and used to quantify diaphragmatic motion.10,17 Both hemidiaphragms can be imaged simultaneously in a transverse-oblique subxiphoid view, which is particularly helpful in the evaluation of paradoxical diaphragmatic motion in cases of diaphragmatic paralysis.3 However, simultaneous visualization of both hemidiaphragms can sometimes be limited on this view due to shadowing from gas within the stomach and transverse colon, necessitating fluoroscopy.
Computed Tomography
Many disorders of the pediatric diaphragm are frequently managed on the basis of radiographs, ultrasound, and/or fluoroscopy alone, including CDH, diaphragmatic paralysis, and diaphragmatic eventration. CT is useful for precisely delineating anatomy and characterizing tissue composition of lesions in selected cases. As discussed in previous chapters, CT produces high-resolution axial images, which can be reformatted and viewed in multiple planes, and postprocessed into threedimensional representations (Fig. 6.7).
CT may be useful in a minority of cases of CDH to better evaluate herniating abdominal viscera.3,9 If performing CT on a patient with CDH, oral contrast should be avoided or used sparingly, as increased bowel distention within the thorax can lead to worsening mass effect on the heart and mediastinal structures. CT plays a much larger role in the evaluation of other disorders of the diaphragm and is the primary modality for evaluation of diaphragmatic tumors14 and traumatic diaphragmatic injury.12
Magnetic Resonance Imaging
MRI is not currently a primary imaging modality utilized in the evaluation of diaphragmatic disease, though this is largely due to high cost and limited availability (Fig. 6.8). Several studies of healthy volunteers have shown that MRI can
accurately depict diaphragmatic motion.18,19,20 Other investigators have shown MRI to be a feasible option for the evaluation and quantification of abnormal diaphragmatic motion in cases of neuromuscular disease.21,22,23 Although ultrasound and fluoroscopy are currently the two main imaging modalities utilized to evaluate diaphragmatic motion, some have advocated for increased utilization of MRI, suggesting that results may be more reproducible than those obtained by ultrasound, a highly operator-dependent modality.24 Most studies investigating MRI of the diaphragm have been performed in adults. As in other applications, MRI may provide a useful alternative to CT and fluoroscopy in some pediatric patients because it spares patients from ionizing radiation.
accurately depict diaphragmatic motion.18,19,20 Other investigators have shown MRI to be a feasible option for the evaluation and quantification of abnormal diaphragmatic motion in cases of neuromuscular disease.21,22,23 Although ultrasound and fluoroscopy are currently the two main imaging modalities utilized to evaluate diaphragmatic motion, some have advocated for increased utilization of MRI, suggesting that results may be more reproducible than those obtained by ultrasound, a highly operator-dependent modality.24 Most studies investigating MRI of the diaphragm have been performed in adults. As in other applications, MRI may provide a useful alternative to CT and fluoroscopy in some pediatric patients because it spares patients from ionizing radiation.
SPECTRUM OF DIAPHRAGMATIC DISORDERS
Congenital and Developmental Anomalies
Diaphragmatic Hernia
A CDH is defined as an abnormal opening in the diaphragm. CDH can be communicating or noncommunicating. Communicating hernias are characterized by a complete opening in the diaphragm with direct communication between the thoracic and peritoneal cavities. Noncommunicating congenital diaphragmatic defects have a membrane that covers the diaphragmatic defect and maintains a separation between the thoracic and peritoneal cavities. The residual membrane in noncommunicating diaphragmatic defects is sometimes referred to as a “hernia sac” or “sac membrane.” A milder condition on the same spectrum as CDH is diaphragmatic eventration, a condition in which the diaphragm is thinned and contains a paucity of muscle fibers. Some authors have advocated for elimination of the terms “hernia sac” and “eventration” because they may lead to confusion.25 Although these terms continue to be widely used in the medical literature (and are used herein), it is important to understand their limitations.
CDHs have traditionally been categorized based on location. The most common location for CDH is the posterolateral diaphragm, classically referred to as a Bochdalek hernia (Fig. 6.9). Bochdalek hernias have been estimated to account for up to 90% of CDHs.26 The second most common location for CDH is the anteromedial diaphragm adjacent to the sternum, classically referred to as a Morgagni hernia (Fig. 6.10). Morgagni hernias have been estimated to account for 9% to 12% of CDHs.2 A recent detailed study of a large collection of pathological specimens has questioned these estimates, showing that up to 36% of CDHs do not occur in one of the classical locations.25 Other locations include the posteromedial, anterolateral, or lateral diaphragm.25 Recognition of this finding has initiated a call for a more precise descriptive classification system for CDH. However, the terms “Bochdalek” and “Morgagni” remain entrenched in the medical parlance. Thus, this book continues to utilize these terms while acknowledging their limitations.
Hiatal hernias also involve the diaphragm, but they differ from CDHs in that they are characterized by herniation of the stomach through the esophageal hiatus rather than through an abnormal opening in the diaphragm (Fig. 6.11).
Bochdalek Hernia
Bochdalek hernias (BHs) are characterized by a defect in the posterolateral diaphragm (Fig. 6.9). Traditional estimates state that BHs account for 90% of CDHs, are left sided in 80% of cases, and have a “hernia sac” in 15% of cases.2,26 However, as detailed in the previous section, the accuracy of these estimates has recently been questioned.25
BHs occur due to abnormal development of the pleuroperitoneal folds in early fetal development.27 BHs are associated with other congenital abnormalities, including congenital heart disease and pulmonary hypoplasia. Pulmonary hypoplasia is often attributed to mass effect on the developing lung from herniated abdominal viscera, but it has been noted that significant pulmonary hypoplasia can be seen even with smaller diaphragmatic defects.25 This realization and genetic analyses have led to the hypothesis that CDH, pulmonary hypoplasia, and cardiac defects all result from a common genetic defect that causes abnormal mesenchymal differentiation in multiple organ systems.28
In current modern practice, most CDHs are initially detected on routine prenatal ultrasound. In one large multicenter study, the average gestational age at the time of diagnosis was 24 weeks.29 In the same study, the detection rate
was 51% when CDH was isolated and 72% when additional congenital malformations were present.29
was 51% when CDH was isolated and 72% when additional congenital malformations were present.29
Imaging findings on prenatal ultrasound may include herniation of bowel loops, stomach, or liver into the thorax.2 Large hernias can cause mediastinal shift.2 Ultrasound parameters including lung-to-head ratio and presence or absence of liver herniation may be used to estimate prognosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree