Stage I
Involvement of a single lymph node region or lymphoid structure such as spleen, thymus, or Waldeyer’s ring
Stage II
Involvement of two or more lymph node regions or lymphoid structures on the same side of the diaphragm. The number of anatomic regions involved should be indicated by a subscript (e.g., II3)
Stage III
Involvement of two or more lymph node regions or lymphoid structures on both sides of the diaphragm. This may be subdivided into stage III1 or stage III2, stage III1 for patients with spleen or splenic, hilar, celiac, or portal node involvement and stage III2 for those with para-aortic, iliac, or mesenteric node involvement
Stage IV
Extensive extranodal disease, beyond the definition of bulky and extranodal disease [4]
Five-year overall survival (OS) is ≥95 in low-risk patients and about 85 % in those at high risk [7]. However, according to some studies, the outcomes are less encouraging, with the 20-year OS as low as 68 %. Event-free survival (EFS) rates are even lower. Younger age at onset seems to be associated with better survival [8].
5.3 Non-Hodgkin’s Lymphoma
5.3.1 Background
NHL accounts for 55 % of childhood lymphomas. The incidence increases dramatically in children between 1 and 3 years of age (from about two cases/million to nine cases/million) and reaches a plateau thereafter, such that in adolescence, NHLs represent about one-third of all diagnosed lymphomas.
The majority of pediatric NHL cases fall into one of four categories: Burkitt’s lymphoma (BL), lymphoblastic lymphoma (LBL), anaplastic large cell lymphoma (ALCL), and diffuse large B-cell lymphoma (DLBCL). Indolent lymphomas composed of small lymphocytes (e.g., small lymphocytic lymphoma, marginal zone lymphoma, mantle cell lymphoma, follicle center cell lymphoma) are extremely rare in children and should be diagnosed with caution.
5.3.2 Staging System and Treatment Strategies
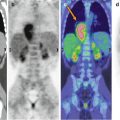
The Ann Arbor staging system does not apply to pediatric NHLs. Indeed, unlike HLs, NHLs spread non-contiguously, with a high frequency of extranodal disease. This pattern prompted researchers to develop a different staging approach, resulting in the St Jude/Murphy’s system [9, 10], in which mediastinal and abdominal localizations are distinct from tumors arising in the remainder of the body and differ in their prognostic significance. Moreover, the classification also considers the extent of surgical excision of abdominal masses (Table 5.2).
Stage I |
A single tumor (extranodal) or single anatomic area (nodal), with the exclusion of the mediastinum and abdomen |
Stage II |
A single tumor (extranodal) with regional lymph node involvement |
Two or more nodal areas on the same side of the diaphragm |
Two single (extranodal) tumors with or without regional lymph node involvement on the same side of the diaphragm |
A resectable primary tumor of the gastrointestinal tract, usually in the ileocecal area, with or without involvement of associated mesenteric nodes only |
Stage III |
Two single tumors (extranodal) on opposite sides of the diaphragm |
Two or more nodal areas above and below the diaphragm |
All primary intrathoracic tumors (mediastinal, pleural, thymic) |
All extensive primary unresectable intra-abdominal disease |
All paraspinal or epidural tumors, regardless of other tumor sites |
Stage IV |
Any of the above with initial involvement of the central nervous system, bone marrow, or both |
Classically, the treatment modality was chosen based on the histological subtype, with treatment intensity modulated according to disease stage and other parameters [11].
At least two major treatment strategies are identifiable for treatment of different NHL subtypes: the first strategy was initially developed for leukaemia and is based on the continuous exposure to cytostatics over a long period of time. It is represented by ten drugs LSA2-L2 (cyclophosfamide, vincristine, methotrexate, daunorubicin, prednisone, cytarabine, thioguanine, asparaginase, carmustine, hydroxyurea) or LSA2-L2 –type protocols. The second strategy consists of shortly repeated, dose-intense combinations of cytotoxic drugs and is represented by the COMP (cyclophosfamide, vincristine, methotrexate, prednisone) or COMP-like regimens. The former has proved to be mostly effective for treatment of precursor NHL (T- and B-LBL), the latter shows the best results in treatment of mature B-NHL (BL, DLCBL) [12]. A summary of treatment modalities for each NHL subtype is provided in specific paragraphs.
5.3.3 Burkitt’s Lymphoma
As the most common NHL subtype in children, BL accounts for up to 40 % of all cases. Three epidemiological variants have been described: endemic, sporadic, and immunodeficiency related [13, 14]. Endemic BL is most commonly seen in male children in equatorial Africa and New Guinea and is strongly associated with EBV infection, which probably leads to the genetic hallmark of endemic BL, the t(8;14)(q24;q32) translocation. This alteration, present in 70–80 % of cases, causes constitutive expression of the MYC oncogene in immunoglobulin-codifying DNA [15]. Sporadic BL is less commonly related to EBV infection and affects both children and adults, with a bimodal age distribution. Immunodeficiency-related BL occurs in the setting of congenital immunodeficiency, HIV infection, and posttransplantation.
The typical presentation of endemic BL is jaw and periorbital swelling, due to the unexplained predilection of EBV for the sockets around the deciduous teeth of young children. The abdomen, and particularly the small bowel, is the most common site of presentation in sporadic and immunodeficiency-associated BL, with symptoms related to obstruction or perforation. Bone marrow infiltration is more common in sporadic BL (~20 % of all cases) than in endemic BL.
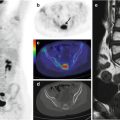
Histologically, BL is diagnosed based on the detection of a monomorphic proliferation of intermediate-sized cells with a proliferative index >95 %. Evenly distributed macrophages containing cellular debris result in a mottled “starry sky” appearance at low magnification (Fig. 5.1b). The immunophenotype is that of a mature surface Ig+ B cell, and both CD20 and CD79a antigens are expressed. The cells are also CD10-positive while terminal deoxynucleotidyl transferase (TdT) expression is lacking.
Localized stages are successfully treated with complete surgical excision followed by two cycles of chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone). Advanced stages warrant between four and eight cycles of chemotherapy, augmented by varying combinations of high-dose methotrexate, cytarabine, and etoposide. Prophylactic intrathecal therapy is also administered to high-risk patients in order to prevent central nervous system (CNS) recurrence.
In developed countries, OS is excellent (>90 %, with differences according to stage at presentation) whereas, regretfully, it is much worse in endemic sub-Saharan countries. The incidence of relapse is highest within 6 months after the completion of treatment and the prognosis of patients who suffer a relapse after optimized treatment is dismal.
5.3.4 T-Cell and B-Cell Lymphoblastic Lymphoma
LBL is the second most common NHL of childhood after BL, comprising 20–30 % of all pediatric NHLs. About 80–90 % of LBLs are of T-cell lineage and present as a bulky mediastinal mass and diffuse systemic disease. LBL of B-cell origin (B-LBL) is less frequent and often limited to the skin, bone, or lymph nodes.
The differential diagnosis of LBL vs. precursor T-cell and B-cell acute lymphoblastic leukemia (ALL) depends on the percentage of blasts in the bone marrow biopsy specimen: LBL is defined as <25 % blasts, while ALL is diagnosed when blasts are >25 % [16]. Tissue involvement is characterized by the diffuse proliferation of small and intermediate cells with dense chromatin and scant cytoplasm; the mitotic rate is frequently elevated (Fig. 5.1c). The immunophenotype of TdT-positive cells with variable expression of the immature T-cell antigens CD1a, CD4, and CD5, together with cytoplasmic CD3 positivity and (usually) surface CD3 negativity, is indicative of T-LBL. By contrast, a phenotype comprising TdT+, CD79+, CD10+, and CD20− is indicative of B-LBL.
The backbone of LBL therapy is the LSA2-L2 regimen and its variants [17–20], which are administered over a period of 18–24 months and divided into induction, consolidation, reintensification, and maintenance phases. Prophylactic intrathecal methotrexate is often sufficient for preventing CNS recurrences such that prophylactic cranial irradiation is progressively disappearing from standard treatments. The OS of patients with limited-stage disease is 85–90 %, while trials of advanced stages have reported 3- to 6-year EFS rates of 70–90 % [16]. The majority of relapses occur within 12–24 months after diagnosis. These patients have poor prognosis, with a 5-year OS of 10 % [11]. Re-induction treatments include platinum-based protocols. Patients with chemosensitive relapses benefit from stem cell transplantation procedures (auto or allo) [21–23].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree