Abstract
Diffuse bilateral confluent air space opacities with air bronchograms may result from alveolar edema, pneumonia, or hemorrhage. Associated findings such as cardiac enlargement and pleural effusions help confirm the diagnosis of congestive heart failure; clinical findings of high fever, elevated white blood count, and productive cough favor pneumonia; and a history of hemoptysis may confirm pulmonary hemorrhage. This appearance is also seen in noncardiac edema from a variety of causes, including near-drowning, drug reactions, or acute respiratory distress syndrome. Pulmonary alveolar proteinosis is one of the few chronic diseases that causes this pattern.
Keywords
acute respiratory distress syndrome (ARDS), alveolar edema, congestive heart failure, diffuse pneumonia, pulmonary alveolar proteinosis, pulmonary hemorrhage, pulmonary vasculitis
Questions
- 1.
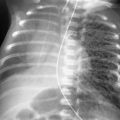
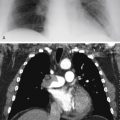
The images in Fig. 15.1, A and B , were of a patient who presented in the emergency department with a known diagnosis of granulomatosis with polyangiitis. Which one of the following complications is most likely?
- a.
Pneumonia.
- b.
Hemorrhage.
- c.
Diffuse alveolar damage (DAD).
- d.
Pulmonary edema.
- e.
Acute interstitial pneumonia (AIP).

Fig. 15.1
- a.
- 2.
Which one of the following is not a sign of air space disease?
- a.
Diffuse coalescent opacities.
- b.
Air bronchograms.
- c.
Acinar nodules.
- d.
Fine reticular opacities.
- e.
Air alveolograms.
- a.
- 3.
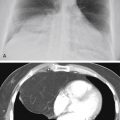
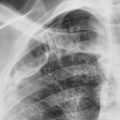
The asymmetric distribution of the diffuse coalescent opacities in Fig. 15.2 is suggestive of which diagnosis?
- a.
Pneumonia.
- b.
Goodpasture syndrome.
- c.
Chronic renal failure.
- d.
Alveolar proteinosis.
- e.
Congestive heart failure.

Fig. 15.2
- a.
- 4.
A 24- to 48-hour delay in the development of pulmonary edema is commonly observed in which of the following conditions?
- a.
Congestive heart failure.
- b.
Pulmonary emboli.
- c.
Smoke inhalation.
- d.
Heroin reaction.
- e.
High-altitude pulmonary edema.
- a.
Discussion
The diffuse air space consolidation 22 , 601 shown in Fig. 15.1, A and B , is a classic appearance and consists of the following: coalescent or confluent opacities with ill-defined borders; butterfly-shaped perihilar distribution; ill-defined nodular opacities around the periphery of the process (“acinar pattern”) 601 , 666 ; and interspersed small lucencies. 457 , 460 Air-filled bronchi surrounded by the confluent opacities are seen as dark branching shadows. These were described by Fleischner 162 as the “visible bronchial tree” and are commonly referred to as air bronchograms 150 (see Fig 15.2 ). The small, interspersed lucent spaces represent groups of air-filled alveoli surrounded by airless consolidated lung. The term air alveologram was applied to these lucent spaces by Felson 150 ; they are the alveolar equivalent of the air bronchogram. The distribution of opacities caused by air space consolidation may be diffuse, lobar, or segmental (see Chapter 14 ). There is a tendency for air space opacities to be labile—that is, changing in severity over a short period of time on serial examinations.
Diffuse ground-glass opacity is occasionally used to describe less opaque, diffuse, confluent opacities seen on chest radiographs, but is more commonly used in reporting high-resolution computed tomography (HRCT). This differs from consolidation in degree of opacity and implies minimal disease. Ground-glass opacities appear on HRCT as gray areas of confluent attenuation that fail to obliterate normal vascular shadows. Ground-glass opacity demonstrated by HRCT results from minimal filling of the alveolar spaces or from thickening of the alveolar walls and septal interstitium. 133 Reticular opacities, whether or not they are demonstrated on a chest radiograph or computed tomography (CT) scan, are not a finding of air space disease. (Answer to question 2 is d .)
Cardiac Pulmonary Edema
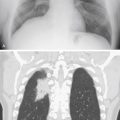
Pulmonary alveolar edema ( Fig 15.3 ) is a classic example of a diffuse air space filling process ( Chart 15.1 ). The presence of alveolar edema, however, does not imply the absence of interstitial edema. Cardiac pulmonary alveolar edema is always preceded by interstitial edema, but the extensive alveolar consolidation obscures the fine reticular opacities of the interstitial process. Radiologic documentation of the underlying interstitial process entails examination of areas not significantly involved by the alveolar filling process. When alveolar pulmonary edema is secondary to congestive heart failure, the alveolar edema often has a perihilar distribution, and Kerley B lines may be present in the costophrenic angles.

- I.
Edema
- A.
Cardiac failure
- B.
Noncardiac (see Chart 15.2 )
- A.
- II.
Exudate (pneumonias)
- A.
- B.
- C.
- D.
- E.
Pneumocystis jiroveci pneumonia (also known as PCP) 69 , 96 , 114
- F.
Parasites (strongyloidiasis) 653
- G.
Aspiration
- H.
Rickettsia (Rocky Mountain spotted fever) 333 , 365
- I.
Tuberculosis 405
- J.
Severe acute respiratory syndrome (SARS) 44 , 70 , 408 , 412
- A.
- III.
Hemorrhage
- A.
Anticoagulation therapy
- B.
Bleeding diathesis (e.g., leukemia)
- C.
Disseminated intravascular coagulation (18- to 72-hour delay) 447
- D.
Blunt trauma 609 (pulmonary contusion, usually is not diffuse)
- E.
Vasculitis
- 1.
Infections (e.g., mucormycosis, aspergillosis, Rocky Mountain spotted fever)
- 2.
Granulomatosis with polyangiitis (formerly Wegener granulomatosis, 441 , 606 classic and variant forms)
- 3.
Goodpasture syndrome 441
- 4.
Systemic lupus erythematosus 441
- 1.
- F.
Idiopathic pulmonary hemosiderosis 163 , 589
- G.
Infectious mononucleosis 595
- A.
- IV.
Other
- A.
Pulmonary alveolar proteinosis 176 , 249 , 457 , 487
- B.
Acute respiratory distress syndrome (ARDS) 130 , 277 , 278 , 291 , 421 , 663
- C.
Acute interstitial pneumonia (AIP) 12 , 257
- D.
Sarcoidosis (very unusual) 386 , 457 , 467
- E.
Mineral oil aspiration (exogenous cholesterol pneumonia)
- F.
Eosinophilic lung disease 85 , 181 , 274
- G.
Chemical pneumonitis
- H.
Drug reactions (see Chart 15.3 )
- A.
The latter sign confirms the underlying interstitial process. Other radiologic signs that may be associated with cardiopulmonary edema and can be helpful in suggesting the diagnosis include: (1) prominence of the upper lobe vessels 454 ; (2) indistinctness of vessels 291 ; (3) peribronchial cuffing 390 ; (4) increased width of the vascular pedicle 390 ; (5) pleural effusion, frequently with fluid in the fissures; and (6) cardiac enlargement with a left ventricular prominence. Correlation of the radiologic findings with clinical findings usually confirms the diagnosis. An electrocardiogram indicating cardiac enlargement or an old or acute myocardial infarction is also supportive evidence, whereas an S3 heart sound, neck vein distention, hepatomegaly, or peripheral edema usually confirm the diagnosis of congestive failure. Also, auscultation over the lungs usually reveals characteristic basilar rales.
Occasionally, alveolar edema is not distributed uniformly. As a result of gravity, when the patient is upright, the edema fluid has a predominantly lower lobe distribution, but when the patient is supine, the fluid tends to have a more posterior distribution. When the patient favors one side, the fluid tends to gravitate to the dependent side. The resolution of pulmonary edema is often not uniform, so that serial chest radiographs reveal a change in the distribution from diffuse perihilar opacities to a pattern of more uneven multifocal opacities. Other causes for atypical or nonuniform distribution of pulmonary edema are usually of pulmonary origin. The best known of these is severe emphysema, which results in a patchy distribution of the alveolar edema. Presumably, loss of vasculature in the emphysematous areas of the lung results in the development of edema in the more normal areas. Pulmonary embolism is a complication of pulmonary edema that may result in a nonuniform or patchy distribution of the alveolar edema. Two factors may determine the distribution of the air space edema following pulmonary embolism: (1) abrupt interruption of perfusion to an area of lung may prevent the development of typical pulmonary edema; and (2) severe ischemia of the lung may give rise to pulmonary hemorrhage. Clinical suspicion of pulmonary embolism in a patient with congestive heart failure will usually require computed tomography angiography (CTA).
Concomitant infection is another cause of uneven distribution of pulmonary edema. Like the diagnosis of pulmonary embolism, this requires correlation with the clinical history. An elevated temperature, leukocytosis, or purulent sputum should prompt a bacteriologic study to rule out superimposed pneumonia.
Cardiac enlargement in combination with diffuse alveolar opacities that are otherwise characteristic of pulmonary edema is not always a reliable indicator that the patient’s primary problem is a cardiac disorder. For instance, chronic renal failure with uremia can cause pulmonary edema (uremic pneumonitis) as well as hypertension and associated heart disease, with the result of cardiac enlargement. Not only does uremia cause true cardiomegaly, which is probably related to chronic hypertension, but it also may cause pericardial effusion. Thus, the pulmonary edema that results from chronic renal failure and uremia is typically associated with enlargement of the cardiac silhouette. Correlation with the clinical history should readily identify uremic pneumonitis.
In contrast to pulmonary alveolar edema and cardiac enlargement, the presence of a normal-sized heart might suggest a noncardiac form of pulmonary edema, but there are situations in which such patients may actually have cardiac pulmonary edema. These include acute cardiac arrhythmias and acute myocardial infarction, which result in pulmonary edema before dilation of the heart. Thus, there are at least two mechanisms for cardiac pulmonary edema with a normal-sized heart.
Noncardiac Pulmonary Edema
The preceding discussion suggests that the radiologic appearance of noncardiac pulmonary edema is similar to that of cardiac pulmonary edema. 546 In general, the most helpful radiologic feature for distinguishing the two is the presence or absence of cardiac enlargement. Accurate assessment of heart size is often difficult. Technical factors—including supine and anteroposterior positioning, especially when done with portable units—may all contribute to cardiac magnification. Patient condition may also lead to inaccurate cardiac size estimation. Patients with emphysema often have cardiac enlargement, although the chest radiograph is suggestive of a normal or even small heart size. Aggressive intravenous (IV) fluid resuscitation may actually enlarge the heart and cause pulmonary edema. The evaluation of serial radiographs is especially useful for distinguishing a number of the causes of noncardiac edema because the evolution of the edema may be strikingly different. Many of the entities listed in Chart 15.2 may result in acute alveolar edema in the absence of the pulmonary vascular and interstitial changes that precede the edema because of either renal failure or cardiac failure. These entities tend to occur in very acute cases of pulmonary edema and are often best diagnosed by clinical correlation, 5 as is shown in the following discussions of acute toxic inhalations, near-drowning, acute airway obstruction, drug reactions, and ARDS.
- I.
Chronic renal failure
- II.
Toxic inhalations
- A.
Nitrogen dioxide (silo filler’s disease)
- B.
Sulfur dioxide 74
- C.
- D.
Beryllium
- E.
Cadmium
- F.
Silica (very fine particles; silicoproteinosis) 299
- G.
Carbon monoxide 549
- A.
- III.
Anaphylaxis (e.g., penicillin, transfusion, 62 radiologic contrast medium 205 )
- IV.
Narcotics (e.g., morphine, methadone, cocaine, heroin) 201 , 473 , 671
- V.
Drug reaction (e.g., interleukin-2 therapy, 90 , 518 β-adrenergic drugs 391 )
- VI.
Acute airway obstruction 422 (e.g., foreign body)
- VII.
Near-drowning 448
- VIII.
High altitude 254
- IX.
Fluid overload
- X.
Cerebral (trauma, stroke, tumor) 467
- XI.
Hypoproteinemia
- XII.
- XIII.
Pancreatitis 494
- XIV.
Amniotic fluid embolism 537
- XV.
Fat embolism
- XVI.
Re-expansion following treatment of pneumothorax or large pleural effusion
- XVII.
Organophosphate insecticide ingestion 339
- XVIII.
Hanta virus pulmonary syndrome 291
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree