General Considerations
Diffuse involvement of the pancreas can occur with various inflammatory, infective, infiltrative, or neoplastic disorders. Any pathologic process that involves the pancreas focally also can cause diffuse involvement ( Box 51-1 ). More common causes of diffuse pancreatic involvement (e.g., pancreatitis) have been discussed previously. This chapter discusses infrequent causes and differential features.
Inflammation
- •
Acute pancreatitis
- •
Chronic pancreatitis
- •
Chronic calcifying pancreatitis
- •
Chronic obstructive pancreatitis
- •
Autoimmune pancreatitis
- •
Infiltration
- •
Cystic fibrosis
- •
Fatty replacement of pancreas
- •
Amyloidosis
- •
Hemochromatosis
Infection
- •
Tuberculosis
- •
Acquired immunodeficiency syndrome
Neoplasm
- •
Lymphoma
- •
Leukemia
- •
Carcinoma
- •
Metastases
Miscellaneous
- •
Nesidioblastosis (persistent hyperinsulinemic hypoglycemia of infancy, congenital hyperinsulinism)
- •
Von Hippel-Lindau disease
Imaging
Imaging can demonstrate the pattern and extent of pancreatic involvement and other ancillary features, which can suggest and facilitate diagnosis. Although imaging features can overlap, differentiation becomes critical from the management perspective. Please refer to Figure 51-1 for an imaging algorithm of diffuse pancreatic diseases.

Differential Diagnosis
Because of overlapping imaging features, clinical and laboratory parameters are often required to elucidate and reinforce the diagnosis. Imaging can guide invasive techniques (biopsy, fine-needle aspiration) in doubtful cases, aid in further substantiation of diagnosis, and guide in treatment planning. Imaging also plays an important role in screening and posttherapy follow-up to demonstrate resolution, recovery, or recurrence. Please refer to Table 51-1 and Figures 51-2 to 51-5 .
| Disease Process | Clinical Data | Specific Laboratory Tests | IMAGING | Imaging Mimics | ||||
|---|---|---|---|---|---|---|---|---|
| Radiography | Ultrasonography | CT | MR | ERCP | ||||
| Acute pancreatitis (see Figure 51-2 ) | Acute presentation ± history of gallstones and/or alcoholism | Serum lipase and amylase | Small bowel ileus | Enlarged, hypoechoic | Enlarged, hypodense Peripancreatic fat stranding and fluid collection | Enlarged, T1-weighted hypointense, T2-weighted hyperintense | — | Lymphoma |
| Chronic calcifying pancreatitis (see Figure 51-3 ) | Chronic presentation History of gallstones, alcohol, ± exocrine and endocrine insufficiency | — | Prevertebral calcification | Small or atrophic, irregular ductal dilatation, ± calcification | Small or atrophic, irregular ductal dilatation, ± calcification | Small or atrophic, MRCP: PD irregularity, beading, dilatation | PD beading, irregular dilatation | Pancreatic CA when associated with focal mass-like lesion |
| Chronic obstructive pancreatitis (see Figure 51-4 ) | Chronic presentation, history of CA or IPMN | — | — | CA: Hypoechoic lesion, small or atrophic pancreas, upstream PD dilatation and irregularity, ± calcification MD IPMN: Diffuse PD dilatation | CA: Hypodense on parenchymal phase of IV contrast, upstream PD dilatation, ± calcification, ± invasion and metastasis MD IPMN: Diffuse PD dilatation ± bulging papilla | CA: Hypointense on parenchymal phase of IV contrast, upstream PD dilatation, ± invasion and metastasis MD IPMN: Diffuse PD dilatation ± bulging major papilla | Bulging papilla with MD IPMN, PD dilated | Mass-like lesion of chronic pancreatitis |
| Autoimmune pancreatitis (see Figure 51-5 ) | Common in males Nonspecific symptoms related to pancreatic involvement | Serum IgG4 | — | Enlarged, hypoechoic | Enlarged, sausage shaped, peripancreatic halo, peripancreatic stranding, PD attenuated or irregular, ± CBD involvement, focal mass-like swelling | T1 hypointense, T2 hyperintense, sausage shaped, peripancreatic halo, peripancreatic stranding, PD attenuated or irregular, ± CBD involvement, focal mass-like swelling | PD attenuated or irregular | Acute pancreatitis, pancreatic CA when associated focal mass-like swelling |
| Tuberculosis (see Figure 51-13 ) | Immigrants, immunocompromised HIV patients Constitutional symptoms, symptoms related to pancreas or other organ system involvement | — | Calcification in chronic or treated cases | Enlarged, hypoechoic, heterogeneity as a result of abscess/calcification | Enlarged, hypodense, heterogeneity as a result of abscess/ calcification/ necrosis, heterogeneous enhancement, peripancreatic edema, peripancreatic, mesenteric, periportal LN, fistulas | Enlarged T1 hypointense T2 hyperintense, heterogeneous enhancement | PD normal, compressed, displaced or stenosed | Carcinoma, lymphoma, AIDS |
| AIDS (see Figure 51-14 ) | High-risk behavior, IVDA | — | — | Enlarged, hypoechoic | Enlarged, hypodense, hemorrhagic necrosis with herpes simplex | Enlarged T1 hypointense T2 hyperintense, heterogeneous enhancement | — | Tuberculosis, carcinoma, lymphoma |
| Lymphoma (see Figure 51-15 ) | Systemic symptoms, jaundice less common | — | Calcification in treated cases | Enlarged, hypoechoic, mesenteric and retroperitoneal LN | Enlarged, hypodense, peripancreatic infiltration, mild homogeneous enhancement, PD dilatation not a feature, mesenteric and retroperitoneal LN extending below renal vein, invasion of other organs, engulfment of vessels Valuable for staging | Enlarged, T1 hypointense, T2 hyperintense, mild homogeneous enhancement, PD dilatation not a feature, mesenteric and retroperitoneal LN extending below renal vein, invasion of other organs, engulfment of vessels | PD normal, displaced, narrowed | Tuberculosis, carcinoma, leukemia, AIDS |
| Leukemia | Systemic symptoms, jaundice not a feature | — | — | Enlarged, hypoechoic, LN, other organ system involvement | Enlarged, hypodense, mild enhancement, LN, other organ system involvement | Enlarged, T1 hypointense, T2 hyperintense, LN, other organ system involvement | — | Lymphoma Carcinoma Tuberculosis AIDS |
| Carcinoma (see Figure 51-16 ) | Middle–elderly age Systemic symptoms, painless jaundice | CA 19-9 for follow-up | — | Enlarged, hypoechoic, LN, vascular invasion, metastases | Enlarged, hypodense, heterogeneity as a result of necrosis/calcification, LN, vascular encasement/invasion, metastases | Enlarged, T1 hypointense, T2 hyperintense, heterogeneity as a result of necrosis/calcification, LN, vascular encasement/invasion, metastases | Irregularity or narrowing | Lymphoma, tuberculosis, AIDS |
| Metastases | Known primary illness, occasionally pancreatic metastases—first sign Systemic and local symptoms | — | — | Enlarged, hypoechoic, LN, involvement of other organs | Enlarged, hypodense, occasionally necrosis, LN, involvement of other organs | Enlarged, T1 hypointense, T2 hyperintense, LN, involvement of other organs | Irregularity or narrowing | Lymphoma, carcinoma, tuberculosis |
| Cystic fibrosis (see Figures 51-6 to 51-10 ) | AR, whites, family history, exocrine and endocrine dysfunction | Sweat chloride test | Homogeneously or heterogeneously hyperechoic, multiple hypoechoic cysts | Size: Normal or atrophic, hypodense as a result of fat/fibrosis, ± calcification, low-attenuation cystic lesions without solid component | Fatty: T1 and T2 hyperintensity Fibrosis: T1 and T2 hypointensity Cysts: T1 hypointensity, T2 hyperintensity | — | Fatty replacement, VHL | |
| Fatty replacement (see Figure 51-11 ) | Advanced age, DM, metabolic syndrome, Cushing’s, long-term use of corticosteroids, chronic pancreatitis | — | — | Echogenic | Hypodense, prominent lobulations | T1 and T2 hyperintense, prominent lobulations | — | Cystic fibrosis |
| Amyloidosis | Chronic or hematologic illness | — | — | Hypoechoic | Hypodense | T1 hypointense and hyperintense, T2 hyperintense | — | Fatty replacement |
| Hemochromatosis (see Figure 51-12 ) | AD, family history, systemic manifestations, endocrine or exocrine pancreatic dysfunction | Serum iron, TIBC, transferrin saturation | — | Normal-appearing pancreas | Hyperdense pancreas and peripancreatic LN | T2 hypointense pancreas and liver | — | — |
| Nesidioblastosis (congenital hyperinsulinism of infancy) | Recurrent hypoglycemia in infancy | Insulin and C peptide | — | Enlarged, hypoechoic | Enlarged, hypodense | T1 hypointense, T2 hyperintense | — | — |
| VHL (see Figures 51-17 and 51-18 ) | Family history, CNS manifestations, endocrine or exocrine pancreatic dysfunction with severe involvement | Genetic testing | — | Multiple hypoechoic cysts, sometimes solid appearance as a result of multiple interfaces, ± SCA | Multiple hypodense cysts with thin wall, ± calcification throughout pancreas ± SCA | Multiple cysts, T1 hypointense, T2 hyperintense ± SCA | — | Cystic fibrosis |




Treatment
Medical management depends on the underlying disease process involving the pancreas and is detailed in the discussion of individual diseases presented in this chapter. Surgical management is generally not recommended for diffuse pancreatic disorders, but palliative measures may be required in some cases.
- •
Overlapping imaging features of diffuse pancreatic disease are seen.
- •
Consideration of clinical, laboratory, and imaging findings are vital for narrowing the differential diagnosis and may help avoid surgery for diagnostic and therapeutic purposes in medically treatable cases.
- •
Histologic diagnosis is crucial before appropriate medical or surgical therapy is instituted.
- •
Confirmation of diagnosis can be done by pathologic evaluation of tissue obtained from the pancreatic lesion or other involved organs.
Infiltrative Disorders
Cystic Fibrosis
Etiology
Cystic fibrosis is a life-threatening disorder caused by mutation in the cystic fibrosis transmembrane conductance regulator gene (CFTR) on chromosome 7.
Prevalence and Epidemiology
Cystic fibrosis is an autosomal recessive disorder that is more prevalent in whites, with an incidence of approximately 1 in 2500 (in this population).
Clinical Presentation
Although predominantly characterized by chronic obstructive lung disease and pancreatic insufficiency, cystic fibrosis is a multiorgan disease affecting the liver, gallbladder, bile ducts, intestines, and the reproductive tract. The clinical and imaging findings vary with severity and duration of disease process in all age groups. Nearly 85% to 90% of patients will present with clinical signs within 1 year of life. Significant loss of pancreatic exocrine cells must be present before development of typical clinical symptoms of insufficiency such as steatorrhea, fat intolerance, failure to thrive, bloating, flatulence, and abdominal pain. Pancreatic function may be preserved in 10% to 15% of patients, and when residual exocrine function is present, patients may present with acute pancreatitis. Endocrine dysfunction is less commonly encountered.
Pathophysiology
The defective transmembrane ion transport in cystic fibrosis leads to accumulation of thick, viscous pancreatic secretions in the pancreatic ducts, leading to ductal ectasia and acinar atrophy. Inflammatory reaction, progressive fatty replacement, fibrosis, and calcification result, with extensive atrophic change occurring in severe long-standing disease. Cyst formation can occur secondary to inspissated secretions obstructing the small pancreatic ducts.
Imaging
The patterns of pancreatic involvement include (1) partial fibrofatty replacement of the pancreas, (2) complete fibrofatty replacement with enlargement of pancreas (lipomatous pseudohypertrophy), (3) pancreatic atrophy without evidence of fatty replacement, (4) diffuse pancreatic fibrosis, and (5) pancreatic cystosis. In pancreatic cystosis, cysts are usually multiloculated with individual cysts measuring 0.5 to 12 cm in diameter. Complete fatty replacement of the pancreas is commonly seen, with fat replacement maintaining the shape of the pancreas. This morphologic finding may be evident in older patients and represents late stage of disease, but it also can be seen earlier with severe involvement.
Radiography.
Calcifications may be seen in the late stage of the disease.
Ultrasonography.
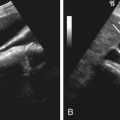
On ultrasonography, the involved pancreas reveals homogeneously or heterogeneously increased echogenicity ( Figure 51-6, A ). The pancreas may be normal or small in size, and the typical fine lobular echotexture of the pancreas is gradually lost. Pancreatic cystosis is seen as multiple, thin-walled, anechoic, multiloculated cysts scattered throughout the pancreas with interspersed hyperechoic pancreatic parenchyma (see Figure 51-6, B ). Ultrasonography is not sensitive for detecting and estimating the extent of pancreatic involvement.

Computed Tomography.
Fatty replacement of the pancreas ( Figure 51-7 ) and fibrosis appears hypodense on computed tomography (CT); measurement of Hounsfield units will differentiate the two processes. A small atrophic pancreas can be seen ( Figure 51-8 ). Calcification, when present, is better appreciated on CT. The cystic lesions appear as well-defined low-attenuation structures without any solid portions or excrescences ( Figure 51-9 ).



Magnetic Resonance Imaging and Magnetic Resonance Cholangiopancreatography.
Fatty changes reveal hyperintense signal Figure 51-10 ), whereas areas of fibrosis show as hypointense signal on T1- and T2-weighted images. Cysts appear as hypointense on T1-weighted images and hyperintense on T2-weighted images. Although magnetic resonance imaging (MRI) is more sensitive in detection of additional small cysts compared to CT or ultrasound, no additional clinically important information is added. Exocrine dysfunction and appearance on MR may not correlate, and a normal-appearing pancreas can be seen in patients with dysfunction. Beading, strictures, poststenotic dilatation, and obstruction of pancreatic duct also may be present and are best characterized on magnetic resonance cholangiopancreatography (MRCP).
- •
Fatty replacement
- •
Cystic changes
- •
Calcifications
- •
Fibrosis
- •
Atrophy
- •
Ductal stenosis and beading

Differential Diagnosis
With cystic changes, differential diagnosis may include cystic pancreatic neoplasms, polycystic kidney disease, and von Hippel-Lindau syndrome. With diffuse fatty change, lipomatosis may be indistinguishable, although clinical findings of recurrent respiratory infections, age, exocrine and endocrine dysfunction, family history, and positive sweat chloride test can aid in diagnosis.
Treatment
Medical Treatment.
Lifelong pancreatic enzyme replacement and insulin therapy are mainstays of treatment in patients with endocrine or exocrine insufficiency.
Surgical Treatment.
End-stage lung disease is treated with bilateral lung transplantation. Excess primary digestive tract malignancies have been reported, and use of immunosuppressive therapy after transplant and exogenous growth hormone can secondarily trigger pancreatic malignancies in patients with cystic fibrosis requiring surgical management.
- •
Genetic testing can result in early diagnosis and treatment.
- •
Although less common, pancreatitis can occur in those with preserved exocrine function.
- •
Asymptomatic patients can be observed with ultrasonography.
- •
Exocrine pancreatic dysfunction may not become clinically apparent until 98% to 99% of pancreatic parenchyma is damaged.
- •
Pancreatic insufficiency may lead to an earlier pseudomonal colonization of airways.
- •
Noninvasive imaging can be useful for quantitative evaluation of morphologic changes in the pancreas and in monitoring the progression of disease, before clinical decline becomes apparent.
Fatty Replacement (Lipomatosis) of Pancreas
Etiology
Fatty replacement of the pancreas is commonly seen with obesity and aging. Metabolic syndrome, characterized by obesity, dyslipidemia, glucose intolerance, hypertension, and proinflammatory or prothrombotic state, is thought to have high association with fatty replacement. Other associated conditions include Cushing’s syndrome, adult-onset diabetes mellitus, chronic pancreatitis, hereditary pancreatitis, alcoholic hepatitis, malnutrition, Shwachman-Diamond syndrome, and long-term use of corticosteroids.
Clinical Presentation
Fatty replacement does not cause clinical symptoms. Clinical presentation is according to the primary disease process.
Pathology
Mature adipocytes and bands of fibrous tissue replace the pancreatic tissue with sparing of acini and islets of Langerhans.
Imaging
Ultrasonography.
The pancreatic gland appears echogenic on ultrasound examination.
Computed Tomography.
Uniform or patchy fatty replacement can be readily identified on CT, which reveals separation of pancreatic parenchyma with prominence of lobulations interspersed with fat. With patchy involvement, the spared regions can be mistaken as pseudotumors. Associated atrophy of the pancreas may be seen to a variable degree, particularly in elderly individuals ( Figure 51-11 ). Massive enlargement of the pancreas due to fatty replacement is known as lipomatous pseudohypertrophy.

Magnetic Resonance Imaging.
Fatty replacement of the gland appears hyperintense on T1- and T2-weighted imaging and will suppress on fat-saturated sequences. MRI may be helpful in distinguishing patchy fatty infiltration and pseudotumor appearance of the residual normal pancreas, whereas true pancreatic adenocarcinoma will rarely demonstrate presence of fat.
- •
Fatty replacement
- •
Prominent lobules
Differential Diagnosis
Clinical diagnosis gives an insight to the cause of fatty replacement. In the presence of clinical diagnosis and imaging findings, no other diagnostic studies are required.
Treatment
No medical treatment is required or available. Sometimes lipomatous pseudohypertrophy can be mistaken as pancreatic mass and taken for surgical resection. Fatty replacement is reversible in obesity after weight reduction, in treated Cushing’s syndrome, and with discontinuation of corticosteroids.
- •
Knowledge of lipomatosis helps recognize this condition and avoids mistaking this benign process from other treatable disorders.
Amyloidosis
Etiology
Amyloidosis is a systemic disorder characterized by abnormal protein folding and extracellular deposition of insoluble fibrillar proteins. Three forms have been defined, with the primary form representing deposition of light-chain immunoglobulins secondary to plasma cell dyscrasias; the secondary form arising as a result of chronic diseases such as rheumatoid, sarcoid, or diabetes; and the familial form representing the autosomal dominant form of the disease with formation of abnormal proteins.
Clinical Presentation
Patients can present with exocrine or endocrine dysfunction or abdominal pain, in addition to the signs and symptoms of associated systemic illness.
Pathology
The pancreas can be involved in primary amyloidosis or, more commonly, as a part of amyloidosis secondary to chronic systemic disease processes. Affinity to Congo red with apple green birefringence under polarized light microscopy are pathognomonic for all amyloidosis.
Imaging
Although literature on characteristic imaging findings on pancreatic amyloidosis is scarce, pancreatic involvement can be focal or diffuse.
Ultrasonography.
The diffusely involved gland appears enlarged and hypoechoic on ultrasound. Often, there is increased affinity to deposition of calcium and punctate calcifications can be seen.
Computed Tomography.
The diffusely involved gland appears enlarged and hypodense on CT. Similarly, punctate calcifications can be seen.
Magnetic Resonance Imaging.
The involved gland appears hypointense or hyperintense on T1-weighted imaging and hyperintense on T2-weighted imaging. Diffuse heterogeneous enhancement may be seen.
Differential Diagnosis
Differential diagnosis for diffuse involvement of the pancreas includes autoimmune pancreatitis, lymphoma, and acute pancreatitis. Involvement of other organ systems can indicate the diagnosis in secondary amyloidosis. Histopathologic evaluation with Congo red and immunostaining of tissue is required for differentiation from other clinical entities.
Treatment
Medical management involves treatment of the systemic disease process in secondary forms of amyloidosis.
- •
Although amyloidosis is a rare cause of diffuse pancreatic involvement, this disease should be considered when chronic or hematologic illnesses coexist.
Hemochromatosis
Etiology
Hemochromatosis is a disorder of excessive iron accumulation.
Prevalence and Epidemiology
Hereditary hemochromatosis is prevalent in Northern European descent and is less common in blacks, Hispanics, and Asian-Americans. The disease is five times more common in men, and they usually experience symptoms at an earlier age. Because women lose iron with menstruation and pregnancy, they tend to store less iron than men. After menopause or hysterectomy, the risk for women is the same as that for men.
Clinical Presentation
Pancreatic involvement manifests as abdominal pain, in addition to the other systemic manifestations of hemochromatosis owing to involvement of heart, skin, liver, thyroid, joints, and other organ systems. Patients will typically present with abdominal pain, fatigue, joint pain, and loss of libido, among other symptoms related to iron deposition in multiple organs.
Pathophysiology
Hemochromatosis can be primary/hereditary or secondary. Hereditary hemochromatosis is an autosomal dominant disorder resulting from excessive absorption of iron; secondary hemochromatosis results from excessive ingestion of iron or from multiple transfusions. Hereditary hemochromatosis is mainly caused by a defect in the HFE gene, which helps regulate the amount of iron absorbed from food. The two common mutations involving the HFE gene are C282Y and H63D . Juvenile and neonatal hemochromatosis, two additional forms, are caused by a mutation in a gene called hemojuvelin.
Imaging
Radiography.
Radiographs have no role in evaluation of pancreatic hemochromatosis. Joint involvement can be separately imaged.
Ultrasonography.
Ultrasonography is noncontributory in most pancreatic cases. Liver involvement may be seen as an altered echotexture or as heterogeneity.
Computed Tomography.
CT characteristically reveals increased density in the pancreas and adjacent peripancreatic lymph nodes, although no correlation has been found between the amount of increased density and pancreatic dysfunction or insufficiency. Unopacified vessels may give a spurious impression of low-density masses in the pancreas, which can be resolved by contrast administration.
Magnetic Resonance Imaging.
Pancreatic involvement is uncommon without cirrhosis. On T2-weighted gradient recalled echo MRI, the liver and pancreas reveal markedly diminished signal intensity compared with that of skeletal muscles ( Figure 51-12 ). Hypointense signal also can be seen on spin echo T2-weighted images, although this sequence is less sensitive. The splenic signal is normal in primary hemochromatosis. On chemical shift in-phase and out-of-phase imaging, reversal of signal drop is seen in comparison to when there is intracytoplasmic fat, with signal drop out seen on in-phase imaging with iron deposition. Quantification of the liver iron content can be performed using T2-weighted gradient recalled echo images. By calculating the ratio of signal intensity of liver to that of fat, mounting iron overload can be serially evaluated.
- •
Hyperdense on CT (pancreas and lymph nodes)
- •
Hypointense on T2-weighted gradient recalled echo MRI

Differential Diagnosis
Systemic involvement of the heart, skin, liver, joints, thyroid, and other organ systems supports the imaging diagnosis. Findings of elevated serum iron concentration, total iron-binding capacity, and transferrin saturation values are characteristic. Liver biopsy is the gold standard for diagnosis.
Treatment
Medical Treatment.
Regular phlebotomy is effective in removing excess iron from the body.
Surgical Treatment.
No surgical management is recommended. However, patients developing hepatic cirrhosis secondary to hemochromatosis are at increased risk for hepatocellular carcinoma, which may require surgical management.
- •
Early identification of disease and appropriate treatment can prevent progression to irreversible pancreatic insufficiency and liver cirrhosis.
- •
Genetic testing can be done for early identification.
- •
Spleen is spared in primary form.
- •
In secondary form, pancreatic involvement occurs only once the reticuloendothelial system becomes saturated with iron (bone marrow, spleen, and liver).
- •
Imaging is performed to determine organ involvement, monitor response to treatment, and diagnose and manage complications such as hepatic cirrhosis and hepatocellular carcinomas.
Infiltrative Disorders
Cystic Fibrosis
Etiology
Cystic fibrosis is a life-threatening disorder caused by mutation in the cystic fibrosis transmembrane conductance regulator gene (CFTR) on chromosome 7.
Prevalence and Epidemiology
Cystic fibrosis is an autosomal recessive disorder that is more prevalent in whites, with an incidence of approximately 1 in 2500 (in this population).
Clinical Presentation
Although predominantly characterized by chronic obstructive lung disease and pancreatic insufficiency, cystic fibrosis is a multiorgan disease affecting the liver, gallbladder, bile ducts, intestines, and the reproductive tract. The clinical and imaging findings vary with severity and duration of disease process in all age groups. Nearly 85% to 90% of patients will present with clinical signs within 1 year of life. Significant loss of pancreatic exocrine cells must be present before development of typical clinical symptoms of insufficiency such as steatorrhea, fat intolerance, failure to thrive, bloating, flatulence, and abdominal pain. Pancreatic function may be preserved in 10% to 15% of patients, and when residual exocrine function is present, patients may present with acute pancreatitis. Endocrine dysfunction is less commonly encountered.
Pathophysiology
The defective transmembrane ion transport in cystic fibrosis leads to accumulation of thick, viscous pancreatic secretions in the pancreatic ducts, leading to ductal ectasia and acinar atrophy. Inflammatory reaction, progressive fatty replacement, fibrosis, and calcification result, with extensive atrophic change occurring in severe long-standing disease. Cyst formation can occur secondary to inspissated secretions obstructing the small pancreatic ducts.
Imaging
The patterns of pancreatic involvement include (1) partial fibrofatty replacement of the pancreas, (2) complete fibrofatty replacement with enlargement of pancreas (lipomatous pseudohypertrophy), (3) pancreatic atrophy without evidence of fatty replacement, (4) diffuse pancreatic fibrosis, and (5) pancreatic cystosis. In pancreatic cystosis, cysts are usually multiloculated with individual cysts measuring 0.5 to 12 cm in diameter. Complete fatty replacement of the pancreas is commonly seen, with fat replacement maintaining the shape of the pancreas. This morphologic finding may be evident in older patients and represents late stage of disease, but it also can be seen earlier with severe involvement.
Radiography.
Calcifications may be seen in the late stage of the disease.
Ultrasonography.
On ultrasonography, the involved pancreas reveals homogeneously or heterogeneously increased echogenicity ( Figure 51-6, A ). The pancreas may be normal or small in size, and the typical fine lobular echotexture of the pancreas is gradually lost. Pancreatic cystosis is seen as multiple, thin-walled, anechoic, multiloculated cysts scattered throughout the pancreas with interspersed hyperechoic pancreatic parenchyma (see Figure 51-6, B ). Ultrasonography is not sensitive for detecting and estimating the extent of pancreatic involvement.