Normal Variants and Congenital Anomalies
The spleen begins to develop during the fifth week of embryogenesis when mesenchymal cells aggregate between the two leaves of the dorsal mesogastrium to form a lobulated embryonic spleen. Rotation of the stomach and growth of the dorsal mesogastrium translocate the spleen from the midline to the left side of the abdominal cavity. The left aspect of the mesogastrium fuses with the peritoneum over the left kidney. Then, rotation of the dorsal mesogastrium establishes a mesenteric connection (the splenorenal ligament) between the spleen and the left kidney. The gastrosplenic ligament is the portion of dorsal mesentery between the spleen and the stomach.
The fetal spleen is normally lobulated. Although such lobulations generally disappear before birth, they may persist along the medial part of the spleen. The notches or clefts on the superior border of the adult spleen are remnants of the grooves that originally separated the fetal lobules. These clefts can be sharp and are occasionally as deep as 2 to 3 cm. They may be erroneously interpreted as splenic laceration in patients with abdominal trauma. The differential points are ancillary findings such as subcapsular hematoma, hemoperitoneum, or a jagged linear area of parenchymal defect.
Accessory Spleen
Etiology
During embryogenesis a failure of the embryonic splenic buds to unite within the dorsal mesogastrium and an extreme lobulation of the spleen with pinching off of splenic tissue may result in accessory spleens.
Prevalence and Epidemiology
An accessory spleen denotes a congenital focus of heterotopic, healthy splenic tissue that is separate from the main splenic body. It is the most common congenital anomaly of the spleen and is detected in 10% to 30% of patients at autopsy and in approximately 16% on computed tomography (CT).
Clinical Presentation
The vast majority of accessory spleens are incidentally discovered without clinical significance, and recognizing them is important so as not to misdiagnose an accessory spleen as lymphadenopathy or as a tumor arising from adjacent organs. In patients with hematologic and autoimmune disorders, the presence of accessory spleens should be noted before planning splenectomy to guide surgeons to remove all functional splenic tissue at surgery, because a remnant accessory spleen after the surgery may evoke relapse of the disorder. An accessory spleen may be noted as a source of “preservable” splenic tissue in the case of rupture of the main splenic body.
Pathophysiology
Accessory spleens are commonly located near the splenic hilum or adjacent to the pancreatic tail ( Figure 60-1 ). However, an accessory spleen also may occur along the splenic vessels, in the gastrosplenic or splenorenal ligament, within the pancreas tail, in the gastric wall, in the greater omentum or the mesentery, or even in the pelvis or scrotum.

Accessory spleens are usually approximately 1 cm in diameter (see Figure 60-1 ) but vary from microscopic deposits not visible on CT to over 4 cm in diameter.
Imaging
When a small accessory spleen is typically located in the splenic hilum there is no confusion at cross-sectional imaging. However, those in atypical locations may be misdiagnosed as various entities according to the location. It is worthwhile to compare their imaging characteristics with those of a normal spleen because accessory spleens invariably exhibit the same features as a normal spleen on various imaging modalities ( Tables 60-1 and 60-2 ; see also Figures 60-1 and 60-2 ). After splenectomy a remnant accessory spleen can be hypertrophied, occasionally mimicking enlarged lymph nodes or another mass ( Figure 60-3 ). In all confusing cases, technetium-99m sulfur colloid ( 99m Tc-SC) scintigraphy is helpful because it can demonstrate functional activity of the splenic tissue.
| Modality | Accuracy | Advantages | Limitations | Pitfalls |
|---|---|---|---|---|
| Radiography | Poor | Limited | Insensitive Nonspecific | Unable to directly visualize splenic abnormality |
| CT | Up to 95% in a case of trauma-related lesion Data of the rest are not available to specify accuracy. | Initial modality of most splenic abnormalities, especially trauma-related lesion | Radiation exposure Adverse effect of contrast agent | Multiple, tiny, diffusely scattered nodules not easy to be detected |
| MRI | Data are not available to specify and compare accuracy of different imaging modalities for evaluation of splenic abnormality | May be additional clue in the difficult case for differentiation | Ineffective in hemodynamically unstable patients High cost Patient cooperation | Small lesions may not be detected |
| Ultrasonography | May be additional clue in the difficult case for differentiation Useful screening method for the splenic injury especially in hemodynamically unstable patient and pediatric patient | Operator dependent | Small lesions may not be detected | |
| Nuclear medicine | Helpful to identify the splenic functional activity of the suspected lesion such as splenosis, accessory spleen, and wandering spleen | Poor spatial resolution | Nonspecific | |
| PET/CT | Radiation exposure High cost | Nonspecific |
| Lesion | Characteristics | CT | MR | Ultrasonography | Distinguishing Clinical Features | Distinguishing Imaging Findings |
|---|---|---|---|---|---|---|
| Accessory spleen | Most common: Congenital anomaly Size: 1 cm Locations: Splenic hilum, adjacent to the pancreas tail | Similar density/signal intensity (T1↓, T2↑)/echogenicity, and contrast-enhancement pattern as normal spleen on unenhanced and postcontrast images | Underlying malignancy (−) Splenic injury or prior splenectomy (−) Normal visceral situs | Typical findings → straightforward diagnosis | ||
| Wandering spleen | Absence of the spleen in its normal position and a soft tissue mass resembling the spleen located somewhere else Location: Left mid abdomen Age: 20-40 years Sex: F > M | No spleen in left subphrenic area Comma-shaped spleen-like mass somewhere else Splenic torsion: Lower attenuation than liver and “rim” sign | A little additional value of MRI | Splenic infarction: Less echogenic Color Doppler: Absence of blood flow | Nonspecific | Absence of the spleen in its normal location and presence of a comma-shaped abdominal or pelvic mass |
| Polysplenia | Multiple spleens or a multilobed spleen Interrupted IVC with continuation to azygos vein Liver: Midline; gallbladder: central location Anomalies of branching pattern of portal vein and biliary tree Truncated pancreas Abnormalities of bowel rotation Age: Early childhood because of the various cardiac anomalies Sex: F > M | Related to the cardiac disease Young patient | Multiple spleens or a multilobed spleen Interrupted IVC with continuation to azygos vein | |||
| ASPLENIA | Absence of splenic tissue on ultrasonography/CT/splenic scan Ipsilateral position of IVC/hepatic veins and aorta Liver: Midline; gallbladder: central location Anomalies of branching pattern of portal vein and biliary tree Truncated pancreas Abnormalities of bowel rotation Very high mortality rate >95% in their first year of life because of severe congenital heart disease Sex: M > F | Splenectomy and trauma (−), and abnormal situs of various organs in thorax and abdomen Related to the cardiac disease | Absence of splenic tissue on ultrasonography/CT/splenic scan Ipsilateral position of IVC/hepatic veins and aorta | |||
| INFECTIOUS AND INFLAMMATORY LESIONS | ||||||
| Bacterial infection | Cause: Hematogenous, trauma, infarction Organism: Aerobic (60%) | Lesion with a low-density center surrounded by a thick, irregular, dense rim | Rarely performed | Poorly defined, hypoechoic or anechoic lesions with irregular boundary | Findings of fever, chills, and leukocytosis helpful | Containing gas: Discriminating finding, but rare |
| Fungal infection | Most common cause: Candida, Aspergillus Size: 5-10 mm Distribution: Diffuse dissemination | Multiple, rounded, low density, small lesion with ring-like enhancement | MR superior to CT Multiple small nodules T1↓ ↔ T2↑ | “Bull’s-eye” pattern: Typical splenic candidiasis | Immunocompromised patients Fever and splenomegaly Nonspecific, sometimes | Diffusely disseminated small nodules: Nonspecific Differential diagnosis: Tuberculosis, sarcoidosis, lymphoma, and metastases |
| Mycobacterial infection | Hematogenous or lymphatic spread, usually from the lung Miliary form, usually | Miliary or multiple small nodules of low density Abdominal lymphadenopathy or peritonitis | Few reports | Tuberculomas: Decreased or mixed echogenicity | Indolent fever, immunocompetence, malignancy (−) and pulmonary abnormality | Diffusely disseminated small nodules: Nonspecific |
| Echinococcosis | Echinococcus granulosus Systemic and intraperitoneal spread from ruptured liver cyst | Spherical, well-defined water-attenuation lesions | Well-defined rounded mass with water-attenuation signal intensity (T1 ↓, T2↑) | Anechoic or mixed echogenic, round lesion | A patient from an endemic area with positive serologic tests | Daughter cysts |
| Sarcoidosis | Multisystem disease Mediastinal and hilar lymph nodes Splenomegaly or multiple small lesions | Normal or enlarged spleen Multiple low-attenuation nodules (1 mm–3 cm) | T1↓, T2↓ | Diffuse increase of splenic echogenicity and splenomegaly | Extraabdominal sarcoidosis and an elevated angiotensin-converting enzyme level | ↓ Signal intensity on T2-weighted MRI |
| VASCULAR LESIONS | ||||||
| Splenic infarction | Most common causes: Embolic occlusion from cardiovascular disease (usually elderly patients) and local thrombosis from hematologic diseases (age <40 yr) | Peripheral wedge-shaped, sharply demarcated area of low attenuation | Of various signal intensity according to age of infarctions | Color Doppler imaging: Absence of blood flow | High-fever (−) Episode of trauma (−) | Peripheral wedge-shaped, sharply demarcated area Differential diagnosis: Splenic abscess, tumor, laceration, and cyst |
| Splenic venous thrombosis | Most common cause: Pancreatitis | Noncontrast CT: High-attenuation lesion Contrast-enhanced CT: Intraluminal, low-attenuation filling defect adjacent to the venous wall, gastrointestinal varices, splenomegaly | Acute thrombus: T1↑, T2↑ | Acute thrombus: Echogenic Color Doppler ultrasonography: Absence of color flow in cases of total occlusion | History of pancreatitis and variceal bleeding | Imaging findings are almost always typical, pinpointing the diagnosis |
| Splenic artery aneurysm | Most incidentally detected Rupture of splenic artery aneurysm is a rare, but life-threatening complication. Sex: F > M | Round to saccular or fusiform dilatation of the splenic artery, with or without peripheral calcification, sometimes with mural thrombus | Various signal intensity according to flow alteration and the extent of the mural thrombus | Anechoic mass with or without peripheral calcification Color Doppler imaging proves the vascular lesion | Asymptomatic, usually | Imaging findings are almost always typical, pinpointing the diagnosis |
| Trauma-related lesions | Most common injured organ after blunt trauma | Best imaging modality for screening of splenic injury Accuracy > 95% Useful for acute hematoma, laceration, active bleeding, pseudoaneurysms, arteriovenous fistula, and posttraumatic infarction | Little role in splenic trauma because of long scanning time | Useful method for the screening of splenic injury, especially in hemodynamically unstable patient and pediatric patient | Episode of trauma: Important clinical data | Splenic cleft may mimic splenic laceration; from lateral surface, fill-in enhancement from periphery on dynamic study |
| Splenosis | Heterotopic splenic implants anywhere Multiple Size: Various | Same echogenicity, density, and signal intensity as normal spleen on ultrasonography, CT, and MRI On dynamic study, similar enhancement pattern as normal spleen | History of splenic trauma or splenectomy is an important clue. Underlying malignancy (−) | 99m Tc-sulfur colloid scintigraphy is helpful to identify the splenic functional activity of the suspected lesion | ||
| Splenomegaly | Most common cause: Portal hypertension | Craniocaudal measurement of 13-14 cm | Clinical presentation: Various | Size measurement, roughly | ||


Radiography.
Accessory spleens are usually not detected by conventional radiographic studies.
Computed Tomography.
CT features of accessory spleens are quite characteristic. They appear as round or ovoid masses with well-defined borders, typically near the splenic hilum or adjacent to the pancreatic tail. Accessory spleens have similar density and contrast-enhancement pattern as a normal spleen on unenhanced and postcontrast CT (see Figure 60-1 ).
Magnetic Resonance Imaging.
On various MR pulse sequences, accessory spleens have the same signal intensities as those of normal spleen (dark on T1-weighted image and bright on T2-weighted and diffusion weighted images) (see Figure 60-1 ).
Ultrasonography.
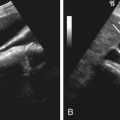
Sonographically, accessory spleens have the same echogenicity and echotexture as the main splenic body (see Figure 60-2 ).
Nuclear Medicine.
The diagnosis of splenic tissue not in its normal anatomic location is best made by 99m Tc-SC scintigraphy, which is highly sensitive and specific for splenic and hepatic tissue.
- •
Typical location near the splenic hilum or adjacent to the pancreatic tail
- •
Same features and enhancement pattern as normal spleen on various imaging modalities
Differential Diagnosis
The differential diagnosis of accessory spleen includes splenosis, polysplenia, peritoneal metastatic implants, lymphadenopathy, and a tumor arising from adjacent organs. The clinical history of previous splenic injury or prior splenectomy permits differentiation of splenosis from other disorders. It is helpful for discrimination of polysplenia that there is bilateral “left-sidedness” of abdominal viscera and cardiovascular anomalies. A previous clinical history of an underlying malignancy such as ovarian cancer or pancreatic cancer helps in differentiating an accessory spleen from peritoneal metastases, lymphadenopathy, and malignant tumor from adjacent organs.
Contrast enhancement of the lesion similar to the spleen on CT, magnetic resonance imaging (MRI), and ultrasonography and intense uptake of tracer by accessory spleens on nuclear scintigraphy permit differentiation of this anomaly from other disorders. The most common location of accessory spleen is the splenic hilum and around the pancreatic tail, whereas splenosis can develop anywhere, including the abdomen, the pelvis, and even the chest. When accessory spleen is located in the pancreas tail, it is important to make a differential diagnosis from neuroendocrine tumor of the pancreas that may appear as a small hypervascular lesion. It has been suggested that diffusion weighted MRI may help differentiate intrapancreatic accessory spleen from solid pancreas tumors, because splenic tissue is usually hyperintense on diffusion weighted images with high b-value.
Treatment
Accessory spleens are normal variants. Treatment is not necessary.
Wandering Spleen
Etiology
Wandering or ectopic spleen refers to migration of the spleen from its normal site in the left upper quadrant to a more caudal location in the abdominal cavity, owing to laxity or maldevelopment of the supporting (gastrosplenic and splenorenal) ligaments. Congenital wandering spleen is characterized by the absence or underdevelopment of one or both of these ligaments, resulting in a long pedicle containing the splenic vessels and, frequently, the pancreatic tail. The acquired form occurs secondary to weakened supporting ligaments from the hormonal effects of pregnancy, abdominal wall laxity, and other causes such as splenomegaly. The long pedicle renders the spleen hypermobile, predisposing it to torsion. Also, wandering spleen is more susceptible to trauma.
Prevalence and Epidemiology
This anomaly is quite rare, with an incidence of less than 0.5% in several large series of splenectomies. It is usually found between the ages of 20 and 40 years and is more common in women. Children make up one third of all cases, with boys and girls younger than the age of 10 years equally affected. The assumed cause in this age group is a congenital defect.
Clinical Presentation
Adult patients with wandering spleen most commonly present with nonspecific abdominal pain associated with a palpable abdominal mass, whereas children most commonly present often with acute abdominal pain. Presumably, pain occurs when there is pressure on the vascular pedicle of the spleen or when torsion develops. Acute splenic torsion may produce clinical signs of acute pancreatitis.
Persistent torsion of greater than 180 degrees may lead to splenic infarction and an acute abdomen. Intermittent and chronic torsion may be related to splenic venous congestion, causing splenomegaly, hypersplenism, fundal gastric varices, and even acute gastrointestinal hemorrhage.
Pathology
Wandering spleen manifests as a mobile mass in the left ( Figure 60-4 ) or central abdomen. It is most commonly located anterior to the left kidney, and the left kidney may be elevated. Splenic flexure of the colon may be medial and anterior to its usual location, or it may be interposed between the diaphragm and the spleen. The stomach may be displaced into the splenic fossa and appear inverted.

Imaging
The most characteristic radiologic finding of wandering spleen is absence of the spleen in its normal position and a soft tissue mass resembling the spleen located somewhere else in the abdomen. The most common location of the spleen is in the left mid abdomen (see Figure 60-4 ).
Radiography.
In patients with wandering spleen, plain radiographs may depict a mobile masslike contour in the left or central abdomen, with a changing location at supine and erect positions.
Computed Tomography.
Wandering spleen is easily diagnosed on CT when there is no spleen in the left subphrenic area and typically a comma-shaped spleen-like mass is found somewhere else in the abdomen (see Figure 60-4 ).
In addition, CT is valuable for the diagnosis of splenic torsion. The twisted splenic pedicle containing splenic vessels, surrounding fat, and sometimes the pancreatic tail may produce the “whirl” sign, representing splenic torsion. In such cases, the splenic parenchyma with congestion or infarction has attenuation considerably lower than that of the liver, more than the usual difference of approximately 10 Hounsfield units (HU). An area of infarction is apparently seen as a perfusion defect on contrast-enhanced CT.
The rim sign, defined as the high density of the splenic capsule compared with the parenchyma, is another CT finding of splenic infarction, which can be noted on both precontrast and postcontrast scans. This finding is probably due to collateral circulation through the short gastric and pancreatic arteries and/or the short gastric and left gastric veins.
Magnetic Resonance Imaging.
MRI may also demonstrate absence of the spleen in the left subphrenic area and a spleen-like mass somewhere else. Although there have been only a few reports on the additional value of MRI in the diagnosis of wandering spleen and splenic torsion up to now, this examination may confer certain advantages over CT in the future, owing to its superior tissue characterization as well as its lack of ionizing radiation.
Ultrasonography.
Ultrasonography may show an abnormal location of the spleen as well. Splenic infarction may cause the spleen to appear less echogenic than normal. Color Doppler ultrasonography may demonstrate the absence of blood flow in the spleen and a high resistive index in the splenic artery in cases of torsion of a wandering spleen.
Nuclear Medicine.
99m Tc-SC scintigraphy is the time-honored method for diagnosing wandering spleen. It depicts the abnormal location of the spleen, which may migrate on sequential studies. A decubitus view may be added to show splenic motion. The absence of uptake in a previously demonstrated wandering spleen indicates torsion.
- •
Absence of the spleen in its normal location
- •
A comma-shaped abdominal or pelvic mass
Differential Diagnosis
When patients with wandering spleen present with acute splenic torsion, physical examination is difficult because of acute pain and clinical signs often mimic those of acute pancreatitis. Also, often the initial impression may be a twisted ovarian cyst, appendicitis, or cholecystitis.
When there is a typical comma-shaped abdominal or pelvic mass with absence of the spleen from its normal location, the diagnosis of wandering spleen on cross-sectional imaging studies can be straightforward. However, in the case of torsion, further diagnostic information on splenic perfusion and viability should be assessed on imaging studies because this information is important for the surgeon, to determine whether splenopexy or splenectomy is the treatment of choice. When the differential diagnosis of wandering spleen from space-occupying lesion is, rarely, difficult, 99m Tc-SC scintigraphy may help for differentiation.
Treatment
For patients without presenting symptoms of torsion, surgical intervention is not advised. In the torsion of wandering spleen, conservative treatment has been employed in selected cases but is not recommended.
The treatment of choice for wandering spleen, especially in young children, is fixation of the spleen (splenopexy). In the presence of splenic infarction, splenectomy is required.
Polysplenia and Asplenia Syndromes
Etiology
Polysplenia and asplenia syndromes represent two major categories in a spectrum of anatomic abnormalities known as visceral heterotaxia. Embryologically altered timing in the development of embryonic body curvature has been postulated as the cause of these visceroatrial sinus abnormalities: accelerated curvature causing polysplenia syndrome and delayed curvature causing asplenia syndrome.
As a cause of embryologic abnormality, genetic mutation in some genes has been reported recently in patients with heterotaxia. Environmental factors also may contribute to heterotaxia.
Prevalence and Epidemiology
Polysplenia syndrome is a multisystemic congenital abnormality characterized by multiple small splenic masses and features of left isomerism. On the contrary, asplenia syndrome refers to congenital absence of the spleen and right isomerism.
Polysplenia syndrome is usually diagnosed in early childhood because of the various and, often, severe cardiac anomalies. There is a female predilection in polysplenia syndrome. Most patients of polysplenia syndrome with severe cardiac anomalies die by the age of 5 years. However, when patients with polysplenia syndrome have a normal heart or only a minor cardiac defect, they reach adulthood asymptomatically without complication and the anomalies may be incidentally found on imaging studies. Therefore, the true incidence and mortality of this disorder are uncertain.
Asplenia syndrome is found in 1 in 40,000 live births and occurs predominantly in males. Congenital heart disease complicates this anomaly in 99% to 100% of patients, accounting for a very high mortality rate of up to 95% in the first year of life.
Clinical Presentation
The mode of clinical manifestation in polysplenia and asplenia syndromes is usually related to the associated cardiac disease. In childhood or adulthood cases of polysplenia syndrome with no or minimal cardiac abnormality, the abdominal visceral abnormalities generally do not produce any symptoms, but bowel malrotation may cause obstruction and pain because of infarction.
Pathology
There is a spectrum of abdominal visceral anomalies as well as cardiopulmonary anomalies in polysplenia and asplenia syndromes.
Spleen.
In the polysplenia syndrome, multiple discrete spleens ( Figure 60-5 ) are considered the hallmark of the disorder. However, the number of splenic masses varies, ranging from many very small spleens to a multilobular spleen with tiny accessory spleens. The spleens may be either left-sided or right-sided and are always seen on the same side as the stomach along the greater curvature. Rarely, one of the multiple spleens may be complicated by torsion with subsequent infarction.

In the asplenia syndrome, the spleen is absent or rarely rudimentary ( Figure 60-6 ).

Liver, Gallbladder, and Biliary Tract.
The liver is mostly situated in the midline, extending symmetrically to both sides of the upper abdomen in both polysplenia and asplenia syndromes. The gallbladder has a central location when the liver is in the midline, and anomalies also may affect the branching pattern of the portal vein (see Figure 60-5 ) and the biliary tree. Biliary atresia may be associated as well.
Pancreas.
Both polysplenia and asplenia syndromes may accompany a truncated pancreas (see Figure 60-5 ), also referred to as short pancreas. Cross-sectional images show only the pancreatic head or the pancreatic head and a small pancreatic body.
Gastrointestinal Tract.
The stomach may be right-sided or left-sided in both polysplenia and asplenia syndromes. There may be abnormalities of bowel rotation; the small bowel is right-sided and the colon is left-sided (see Figure 60-5 ). An inverse relationship between the superior mesenteric artery and the superior mesenteric veins is also frequently noted.
Venous System.
The most common venous anomaly associated with polysplenia syndrome is interruption of the inferior vena cava (IVC) with azygos or hemiazygos continuation, occurring in 65% to 80% of individuals with polysplenia (see Figure 60-5 ). Caudad to the caval interruption, the IVC may be right or left of the aorta or may be duplicated. The hepatic segment of the IVC is often absent, and the hepatic veins drain directly into the right atrium. A preduodenal portal vein is another common venous anomaly.
In asplenia syndrome, IVC interruption with azygos continuation or hemiazygos may be encountered but is very rare. An ipsilateral location of the aorta and IVC has been reported to be a consistent finding in asplenia. However, this finding is not always present in all patients. The abdominal aorta may be located slightly to the right of midline and the IVC slightly to the left to midline along their entire lengths (see Figure 60-6 ).
Cardiac and Pulmonary System.
Most patients with polysplenia or asplenia syndrome have cardiac abnormalities. In comparison with asplenia syndrome, serious cardiac malformation is less common in patients with polysplenia syndrome. Patients with polysplenia syndrome have a high frequency of bilateral bilobed lungs and bilateral hyparterial bronchi (pulmonary artery courses over ipsilateral bronchus). In most patients with asplenia, the lungs are bilaterally trilobed and the bronchi are located over the ipsilateral pulmonary artery (eparterial bronchi).
Imaging
Radiography.
Heart size in asplenia syndrome is usually normal or small on chest radiography. Cardiac enlargement mostly indicates cardiac valvular disease. A symmetric liver shadow (see Figure 60-6 ) and ectopic location of gastric gas may be noted as well. A right-sided bronchial pattern (see Figure 60-6 ) and a minor fissure may be present bilaterally. Both pulmonary arteries project anterior to the trachea on lateral chest radiographs. Superior mediastinal widening may result from a bilateral superior vena cava.
Radiographic findings in patients with polysplenia syndrome depend on which heart defect is present. If there is an interruption of the IVC, the shadow of this vessel is absent on the lateral chest radiograph and the extension via the enlarged azygos vein may cause mediastinal widening in the frontal view. In polysplenia, bilateral symmetric bilobes with usually bilaterally hyparterial bronchi may be seen.
Computed Tomography.
Analyzing the shape and location of the heart, liver, gallbladder, bowel, and spleen on contrast-enhanced CT is useful in determining the situs abnormalities.
Magnetic Resonance Imaging.
Like CT, abdominal MRI can be helpful to evaluate abdominal situs abnormalities. In addition, MRI may be a useful diagnostic tool for diagnosing cardiovascular anomalies in polysplenia and especially asplenia syndromes.
Ultrasonography.
Although limited in that it is operator-dependent, ultrasonography may demonstrate the abdominal situs abnormalities without radiation hazard and intravenous use of a contrast agent and even in critically ill patients.
Nuclear Medicine.
Spleen scans using various agents including 99m Tc-SC and tagged red blood cells are used in detecting splenic tissue. However, with advances in cross-sectional radiologic studies, these radioisotope studies are less frequently used than previously. Moreover, even in the presence of multiple spleens in polysplenia syndrome, splenic function may not be normal, which limits the value of radioisotope studies.
Differential Diagnosis
The differential points of asplenia or polysplenia from splenectomy and splenosis are the previous history of splenectomy and splenic rupture after abdominal trauma.
Treatment
Treatment planning depends on the wide spectrum of polysplenia and asplenia syndrome. In contrast to polysplenia, asplenic patients need prophylactic antibiotics. Cardiac malformations may require surgical correction.
Infectious and Inflammatory Lesions
Bacterial Infection
Etiology
A pyogenic splenic abscess is most commonly caused by the hematogenous spread of infection (75%), followed by penetrating trauma (15%), and prior splenic infarction (10%). Approximately 60% of pyogenic splenic abscesses are due to aerobic organisms, such as Staphylococcus, Streptococcus, Escherichia coli, and Salmonella . Anaerobic organisms are found in 6% to 18% of cases, with Bacteroides being the most common.
Prevalence and Epidemiology
Although splenic abscess was once considered rare, its frequency of occurrence has grown over the decades as a result of an increasing number of immunosuppressed patients because of aggressive chemotherapy, bone marrow or organ transplantation, and acquired immunodeficiency syndrome (AIDS). Immunosuppressed patients make up 24% to 34% of patients with splenic abscesses.
Clinical Presentation
Fewer than half of patients with splenic abscesses classically present with fever and chills, left upper quadrant pain and tenderness, and splenomegaly. Left pleural effusion and consolidation may be found in one third of patients. Laboratory findings are nonspecific, with leukocytosis the most common. Complications such as rupture and subsequent subphrenic abscess and peritonitis may result from a delayed diagnosis and have a high mortality rate.
Pathology
There is no site of predilection of splenic abscesses, but multiple lesions are typically centrally located. Splenic abscesses appear solitary and unilocular (65%), solitary and multilocular (8%), or multiple (27%).
Grossly, splenic abscess can be seen to have irregular borders and no capsule or pseudocapsule. At microscopic examination, suppuration or liquefactive necrosis can be seen, depending on the time course, within the abscess.
Imaging
Radiography.
The most frequent plain radiographic finding in patients with splenic abscess is a left pleural effusion. Mottled air and extraluminal air/fluid levels in the left upper quadrant also may be seen, but these findings are rarely encountered.
Computed Tomography.
With its high sensitivity (96%), CT is the noninvasive diagnostic method of choice for splenic abscesses. On CT, pyogenic splenic abscess appears as a lesion with a low-density center (fluid, necrotic tissue, or pus) surrounded by a thick, irregular, dense rim ( Figure 60-7 ). Although CT can be diagnostic if the intrasplenic fluid collection contains gas, gas is present in only a small number of splenic abscesses ( Figure 60-8 ). Splenic abscess also can contain septa that vary in thickness from 1 to 10 mm. A wedge-shaped abscess may be seen in patients with endocarditis and associated septic emboli.


Magnetic Resonance Imaging.
MRI is rarely performed in patients with suspected splenic abscesses because CT is highly sensitive, and the condition of many patients is not considered clinically stable. MRI shows the abscess as a lesion of fluid signal intensity, with low and high signal intensities on T1- and T2-weighted images.
Ultrasonography
Although ultrasonography is useful as a screening examination for the splenic abscesses in bedridden or dyspneic patients, it may be technically difficult because of ileus, overlying ribs, and perisplenic fluid. Most abscesses appear as poorly defined, hypoechoic or anechoic lesions with irregular boundaries (see Figure 60-7 ). If gas is present, high echogenicity combined with “dirty” shadowing can be demonstrated. Abscesses are typically avascular on color Doppler imaging.
Nuclear Medicine.
If an abscess is 2 cm or greater in diameter, it can be demonstrated as a nonspecific splenic filling defect on 99m Tc-SC scintigraphy.
Positron Emission Tomography With Computed Tomography.
Most splenic abscesses exhibit abnormal uptake of fluorodeoxyglucose (FDG), because activated leukocytes show increased glucose uptake.
Differential Diagnosis
When the differentiation of splenic abscesses from other focal lesions is difficult, clinical findings of fever, chills, and leukocytosis are helpful.
The differential diagnosis of focal low-density and hypoechoic lesions is extensive and includes splenic infarction, cysts, tumors, and hematomas. Splenic infarction appears as a wedge-shaped lesion in a peripheral location, with no contrast enhancement. Splenic hematoma has a history of abdominal trauma and hemoperitoneum. Splenic tumors such as lymphoma may have findings similar to those of abscesses. If the intrasplenic low-attenuation lesion contains gas, it can be diagnostic of a splenic abscess.
Treatment
Patients with pyogenic splenic abscesses should have proper antibiotic treatment. Splenectomy is indicated when there is persistent bacteremia and a lack of response to medical therapy. Recently, image-guided percutaneous drainage of the abscesses is becoming the standard of treatment.
Fungal Infection
Etiology
The most common fungal agents involving the spleen are Candida, Aspergillus, and Cryptococcus.
Prevalence and Epidemiology
Fungal organisms are found in 26% of splenic abscesses, and they are found almost exclusively in immunocompromised patients. For example, fungal infections occur in 40% of patients with hematopoietic malignancies such as leukemia. The use of aggressive immunosuppressive agents in numerous diseases places an increasing population at risk.
Clinical Presentation
The clinical diagnosis of fungal infection is often difficult because the manifesting symptoms may be similar to those of the patient’s primary disorder—fever and splenomegaly. Although the definite diagnosis requires microbiologic evidence of fungal infection, the result of blood culture may be negative in 50% of these patients. Therefore, when there is a strong clinical suspicion, a negative culture result does not rule out the diagnosis of fungal infection. Disseminated fungal infection carries a significant morbidity and mortality. Without prompt treatment, the infection is often fatal.
Pathology
Most splenic fungal infection is disseminated diffusely in the spleen. Fungal microabscesses are typically 5 to 10 mm ( Figure 60-9 ) and seldom larger than 2 cm.

Gross examination of splenic fungal infection may show innumerable small (<5 mm in diameter) fungal deposits in the entire spleen (see Figures 60-9 and 60-10 ). At macroscopic examination, concentric rings can be seen with the central necrotic hyphae; these rings are surrounded by viable hyphae and a rim of peripheral inflammation.

Candida is the fungal organism most frequently encountered. Splenic candidiasis may result from colonization of the gut, which locally disseminates after the onset of neutropenia and mucosal damage. Besides the spleen, multiorgan involvement is common. The gastrointestinal tract is almost invariably involved, and esophageal candidiasis is especially common.
Imaging
In immunosuppressed patients with unexplained clinical deterioration, imaging studies should be promptly performed to search for the microabscesses, because the clinical diagnosis of splenic fungal infection is often difficult. Imaging workup should include both CT and ultrasonography to maximize sensitivity. Use of a high-frequency transducer may be helpful to detect and monitor a small splenic lesion in a patient with suggested fungal infection, especially a child (see Figures 60-9 and 60-10 ).
Radiography.
Radiographically, acute splenic enlargement may be the sign of splenic fungal infection.
Computed Tomography.
On CT, the most common findings are multiple rounded areas of low density (see Figures 60-9 and 60-10 ) or calcifications. Ringlike enhancement may increase the conspicuity of some lesions on postcontrast CT.
Magnetic Resonance Imaging.
It has been proposed that MRI is superior to CT for the detection of fungal microabscesses. These lesions are multiple, small, and of intermediate or low signal intensity on T1-weighed images and of high signal intensity on T2-weighted images.
Ultrasonography.
Four sonographic patterns of splenic fungal abscesses have been described: (1) the “wheel within a wheel” appearance, (2) “bull’s-eye” appearance (see Figure 60-9 ), (3) hypoechoic lesions (see Figure 60-10 ), and (4) echogenic foci with variable posterior acoustic shadowing. The wheel within a wheel pattern is seen with early disease and consists of a central hypoechoic nidus, inner echogenic zone, and peripheral hypoechoic zone, corresponding to central necrosis, surrounding inflammation, and fibrotic ring, respectively. The most typical and specific sonographic pattern of splenic candidiasis is the bull’s-eye pattern (see Figure 60-9 ). The other two patterns are nonspecific.
Nuclear Medicine.
99m Tc-SC scintigraphy is often falsely negative in cases of microabscesses.
Positron Emission Tomography With Computed Tomography.
Some cases of invasive candidiasis and cryptococcosis in the spleen have been reported that show multiple focal areas of increased activity of FDG on PET. The researchers asserted that, although not meant as a first-choice examination, FDG-PET should be considered in difficult cases for more accurate staging and treatment monitoring of infections.
Differential Diagnosis
Clinically, it is difficult to differentiate splenic fungal infection from the aggravation or relapse of patient’s primary disorder such as leukemia because the manifesting symptoms such as fever and splenomegaly may be nonspecific and similar to each other.
Radiologically, splenic fungal microabscesses should be differentiated from metastases, lymphoma, and disseminated mycobacterial infection. However, the classic clinical findings of fever and neutropenia and a history of chemotherapy and use of broad-spectrum antibiotics suggest the diagnosis of fungal microabscesses.
Treatment
Although it is desirable to ascertain the microbiologic evidence of splenic fungal infection before treatment, it is not always possible. Therefore, some investigators have concluded that empirical antifungal therapy should be administered to all neutropenic patients who have positive imaging findings. Amphotericin B is a traditional antifungal agent, and fluconazole and various new azoles are becoming available for the management of systemic fungal infection.
Splenectomy is indicated when there is no response to medical antifungal therapy.
Mycobacterial Infection
Etiology
Abdominal infection with Mycobacterium tuberculosis may develop as a result of hematogenous or lymphatic dissemination from a distant focus, usually from the lung.
Prevalence and Epidemiology
Splenic tuberculous involvement is found in 80% to 100% of cases of disseminated, miliary pulmonary tuberculosis at autopsy. Immunodeficiency associated with alcoholism, intravenous drug abuse, diabetes, cancer, corticosteroid therapy, or AIDS is a risk factor. Because untreated abdominal tuberculosis carries a 50% mortality rate, early diagnosis and treatment are essential.
Clinical Presentation
The various clinical manifestations of splenic tuberculosis include fever, hepatosplenomegaly, ascites, and, rarely, hypersplenism. Although a history or radiographic evidence of pulmonary tuberculosis may be suggestive, nearly one fifth of patients with abdominal tuberculosis have no evidence of extraabdominal disease.
Pathology
Infection of the spleen with M. tuberculosis usually occurs in a miliary form. The first response to tuberculous bacilli is a localized acute inflammation in the site where the largest amount of lymphoid tissue is located. After 2 to 3 weeks, a tubercle with epithelioid cells forms, surrounded by lymphatics (miliary form). Caseous necrosis of the tubercle begins after 2 to 4 weeks, and fibrous scarring may follow (tuberculoma). In patients with AIDS, however, the absence of a granuloma does not rule out tuberculosis because caseating granuloma formation occurs infrequently. Viable mycobacteria pass into the intramural lymphatics and reach the regional lymphatics and lymph nodes, where tuberculoma formation continues.
Imaging
Radiography.
The common findings in radiographic examination include mild splenomegaly, hepatomegaly, and pleural effusion.
Computed Tomography.
Splenic tuberculosis appears as miliary or multiple small nodules of low density ( Figure 60-11 ). In active infection, CT may also reveal abdominal lymphadenopathy, often with low central attenuation ( Figure 60-12 ). High-attenuation ascites with nodular peritoneal thickening and involvement of liver with hepatomegaly or focal lesions also may be noted. Treated splenic tuberculosis exhibits calcium deposition, representing healed, calcified granulomas ( Figure 60-13 ) that appear as scattered, discrete, small calcifications in the normal-sized spleen.