The value of magnetic resonance imaging (MRI) for evaluating a variety of gynecologic diseases, owing to its excellent tissue contrast-reflecting pathology, has been established. Diffusion-weighted (DW) imaging allows the observation of differences in molecular diffusion caused by the random and microscopic motion of molecules (“brownian motion”). Recent technical advances in DW imaging greatly enhanced the clinical value of MRI of the body. DW imaging plays an important role in the diagnosis of brain disorders, more specifically diagnosis of acute stroke. It has not been fully applied to body MRI because the images become more distorted by its sensitivity to motion, resulting in misregistration attributable to chemical-shift artifacts. DW imaging can demonstrate abnormal signals emitted by pathologic foci based on differences in molecular diffusion. DW imaging can delineate malignant lesions displaying hyperintense signal because water diffusion is restricted in tissues of high cellularity. It also permits the quantitative evaluation of the apparent diffusion coefficient (ADC) that may be helpful for monitoring therapeutic outcomes. Decreased ADC values of malignant tumors compared with those of normal tissues or benign lesions have been previously reported. DW imaging has a potential role in distinguishing cancerous from normal tissues.
Description of Technical Requirements
DW imaging is obtained by measuring signal loss after a series of two motion-providing gradient pulses are added to both sides of a 180-degree refocusing radiofrequency (RF) pulse to enhance differences in molecular diffusion between tissues. DW imaging estimates the mean distance traveled by all hydrogen nuclei in every voxel of imaged tissue. The greater this mean distance, the more self-diffusion of water molecules has occurred in a certain time interval. The most common method used in clinical MRI is bipolar-gradient diffusion preparation with single-shot echo-planar imaging. The latter approach can be limited by susceptibility artifacts but can capture the diffusion contrast while minimizing both eddy current and motion artifacts. Recent developments in parallel imaging techniques have improved the quality of body DW imaging by reducing the acquisition time and by minimizing the echo-planar imaging-related susceptibility artifacts.
Techniques
Indications
In the female pelvis, DW imaging can be applied for tumor detection to differentiate between benign and malignant lesions and for the detection of lymph node and bone metastases. DW imaging may also have a potential role in monitoring therapeutic outcome.
Contraindications
There are no contraindications for a DW imaging sequence in the female pelvis.
Technique Description
DW imaging provides quantification of brownian motion of water protons by calculating the ADC, and can be used for in vivo quantification of the combined effects of capillary perfusion and diffusion. The degree of restriction to water diffusion in biologic tissue is inversely correlated to tissue cellularity and the integrity of cell membranes. Free motion of water molecules is more restricted in tissues with a high cellular density. DW imaging with single-shot echo-planar imaging can provide excellent contrast-to-noise ratio because the signals of most organs are low, whereas the lesion signals remain high. The sensitivity of the DW imaging sequence to water motion can be varied by changing the gradient amplitude, expressed as the b -value. By performing DW imaging using different b -values, quantitative analyses can be performed to determine the apparent diffusion coefficient. Because diffusion in tissue is limited by cellular structures, to establish a reliable estimate of the mean distance traveled by the hydrogen nuclei, DW imaging is acquired in at least three orthogonal directions for each b -value. This phenomenon of varying restriction of self-diffusion along different axes is called anisotropy . As in linear aligned tissue, this anisotropy is more pronounced because there is one direction that contributes most to the diffusion. Diffusion tensor imaging is a technique that quantifies the level of anisotropy in tissue, expressed in a fractional anisotropy value. The latter can be used in addition to DW imaging to determine the structural organization of tissue along which diffusion takes place.
Pitfalls and Solutions
DW imaging typically has T2-weighted and DW characteristics. The intensity of the signal on the DW image represents a combination of signal from the T2 relaxation and the dephasing caused by the water motion in the presence of diffusion gradients. At low b -values (<100 s/mm 2 ) there is greater contribution from the T2 signal, and at high b -values contrast is determined by relative diffusion. When a diffusion image is bright because of high T2 signal rather than restricted diffusion, it is known as T2 shine-through effect. On DW imaging with an intermediate b -value (e.g., 500 s/mm 2 ), urine or ascites appears as high-signal comparable to tumor. ADC maps should be obtained with at least two b -values, typically a low b -value (50 to 100 s/mm 2 ) and a high b -value (>500 s/mm 2 ). Tissue microperfusion can contaminate the signal attenuation in DW imaging acquisition, which can be decreased by choosing a low b -value greater than 0 s/mm 2 (preferably ≥50 s/mm 2 ).
To minimize the influence of bulk motion as a distorting factor and minimizing T2 shine-through, typically an echo time as short as possible, a smaller number of echo train lengths, and application of a parallel imaging technique are chosen. Although a wider receiver bandwidth reduces the signal-to-noise ratio, it is recommended because it shortens the duration of acquisition of the MRI signal and reduces susceptibility artifacts.
Ghosting from respiratory motion and chemical shift artifact can be reduced by fat suppression. The method of fat saturation can significantly influence the signal-to-noise ratio of the image and repetition time. In body regions, short time inversion recovery (STIR) fat suppression technique may provide more homogeneous fat suppression compared with chemical shift selective fat suppression. Consequently the signal-to-noise ratio STIR fat suppression technique may be prolonged because of the longer repetition time and an increased number of excitations, which is required for decreased signal-to-noise ratio.
Image Interpretation
There are two methods for assessment of DW imaging: (1) visual assessment of the DW images and ADC maps; and (2) quantitative assessment of ADC values, which gives more specific information regarding molecular diffusion. The ADC value can be derived on a voxel-by-voxel basis and depicted on an ADC map, which allows one to measure the ADC values of a specific region of interest.
Malignant tumors are generally depicted as foci of increased intensity on DW imaging because water diffusion is restricted in highly cellular tissue in malignant tumors. A variety of benign tumors, however, can show restricted diffusion, which may limit the role of DW imaging. Hence the ADC maps and DW images should be read in conjunction with the conventional MRIs. On DW imaging with a high b -value (i.e., >1000 s/mm 2 ), malignant tumors and lymph nodes are more conspicuous compared with images with lower b -values because most of the normal pelvic tissue is strongly suppressed.
Uterine Cervix
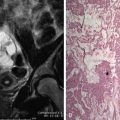
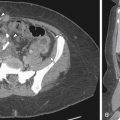
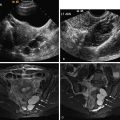
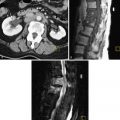
Normal cervical stroma contains large amounts of fibrous tissue, so that it demonstrates low signal intensity on T2-weighted MRIs and delayed enhancement on postgadolinium T1-weighted MRIs. Because of the greater cellularity of cervical cancer compared with the normal fibroelastic cervical stroma, tumor demonstrates higher signal intensity on T2-weighted images and greater enhancement on postgadolinium T1-weighted MRIs. The ADC of cervical cancers is significantly lower than the median ADC of normal cervix stroma, with little overlap. Cervical cancer has been shown to demonstrate impeded diffusion relative to normal cervical stroma. Examples of cervical cancer images using diffusion imaging are seen in Figures 6-1 through 6-3 . Distinction between histologic tumor grades and subtypes based on ADC is not possible. This may be the result of factors such as nuclear size and necrosis. ADC may have predictive value in squamous tumors. Furthermore, the mean ADC value of cervical cancer has been reported to be significantly increased after chemotherapy and/or radiation therapy. Further study into its predictive value for long-term outcome will determine the ultimate clinical value.