List of Abbreviations
ADC
apparent diffusion coefficient
BI-RADS
Breast Imaging Reporting and Data System
BPS
background parenchymal signal
DCE
dynamic contrast-enhanced
DCIS
ductal carcinoma in situ
DWIST
Diffusion-Weighted Magnetic Resonance Imaging Screening Trial
DW MRI
diffusion-weighted MRI
EPI
echo-planar imaging
MIP
maximum intensity projection
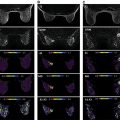
Although dynamic contrast-enhanced magnetic resonance imaging (DCE MRI) is highly sensitive and endorsed by multinational organizations as a supplemental screening tool for high-risk women, widespread implementation of DCE MRI is limited by high cost and uncertain long-term effects of gadolinium retention from contrast administration. In addition, the cost-effectiveness of DCE MRI in intermediate risk patients, such as those with history of breast cancer and dense breasts, remains unclear. Therefore there is great interest in identifying an affordable, unenhanced imaging modality suitable for breast cancer screening. Diffusion-weighted (DW) MRI has emerged as one of the leading options, owing to its short scan time, relative availability and promising sensitivity for identifying breast cancer. DW MRI enables detection of breast malignancies without the need for administering a contrast agent, based instead on microstructural characteristics (e.g., cellular density), as reflected by endogenous diffusional water movement ( Fig. 6.1 ). To date, most experimental and clinical uses of DW MRI have been as an adjunct to DCE MRI in lesion assessment, for preoperative staging of ipsilateral and contralateral breasts, and for evaluating the response to neoadjuvant chemotherapy. However, there is increasing interest in exploring the use of DW MRI as a stand-alone tool for breast cancer detection.
This chapter summarizes the evidence for DW MRI in cancer detection and describes the optimal unenhanced breast cancer screening methods. In addition, the chapter discusses ongoing multicenter DW MRI screening trials and issues associated with clinical implementation.
Current Evidence for DW MRI as a Stand-Alone Modality
Real-world performance of DW MRI for noncontrast cancer detection in the clinical screening setting has been investigated in a variety of reader studies, most of which were performed retrospectively. The readers in these studies assessed only unenhanced MRI sequences (i.e., DW MRI with or without anatomical nonenhanced T1- or T2-weighted sequences) for suspicious findings and were blinded to DCE MRI. They assigned either a binary category (suspicious vs. benign/negative) or a number on a scale corresponding to the level of suspicion, similar to the Breast Imaging Reporting and Data System (BI-RADS) categories. Study designs ranged from inclusion of only asymptomatic intermediate- to high-risk patients, patients with suspicious imaging or clinical symptoms, to those with known malignancy; some studies included a combination of more than one of the above.
DW MRI cancer detection performance across these various studies is summarized in Table 6.1 . The mean sensitivity was 81% (range 44%–97%), and the mean specificity was 88% (range 73%–96%). However, among studies that simulated clinical screening experience by including negative/benign cases, mean sensitivity was 76% (range 45%–100%), and the mean specificity was 89% (range 79%–95%). Variation in the reported sensitivities is likely due to the inclusion criteria, imaging, and interpretation protocol. The study with the lowest sensitivity included only mammographically occult cancer and used relatively low maximum b values (600–800 s/mm 2 ), whereas some studies with higher sensitivities evaluated only (previously biopsied) known malignancy, used advanced imaging acquisition techniques, or performed double reading.
| Study | Total Women | Cancer Prevalence † | Field Strength (tesla) | Max b Value(s/mm 2 ) | MRI Sequences Evaluated | Study Population | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|
| 70 |
| 1.5 | 1000 | ssEPI, STIR, ADC | Known malignancy | 97 | N/A | |
| 48 |
| 1.5 | 800 | ssEPI, T1WI, T2WI | Known malignancy | 94 | N/A | |
| 80 |
| 1.5 | 1000 | ssEPI, T2WI, ADC ‡ | Suspicious mammographic or ultrasound findings and/or clinical symptoms |
|
| |
| 63 |
| 1.5 | 1000 | ssEPI, T2WI | DCE MRI detected asymptomatic malignancy + negative controls | 50 | 95 | |
| 46 |
| 1.5 | 800 | ssEPI, T2WI ADC ‡ | Under 50 years of age with known malignancy + negative controls | 74 | 93 | |
| 58 |
| 3 | 750 | ssEPI, T2WI | Suspicious mammographic or ultrasound findings of <2 cm |
|
| |
| 67 |
| 1.5 | 1000 | ssEPI, T1WI, STIR, ADC | Known malignancy, patients with suspicious mammographic or ultrasound findings, and intermediate- to high-risk screening |
|
| |
| 280 |
| 1.5 | 1000 | DWIBS, T2WI, STIR, ADC ‡ | Suspicious mammographic or ultrasound findings and high-risk screening | 94 | 79 | |
| 50 |
| 1.5 | 1500 | DWIBS MIP, T2WI | Suspicious mammographic or ultrasound findings | 92 | 94 | |
| 118 |
| 1.5 | 1000 | ssEPI, STIR, ADC ‡ | Known malignancy and patients with suspicious mammographic or ultrasound findings |
|
| |
| 61 |
| 1.5 | 1150 | ss-EPI | Suspected breast pathology | 44 | – | |
| 87 |
| 3 | 1000 | rs-EPI, T1WI, rs-EPI fused to T1WI, ADC ‡ | Known malignancy |
|
| |
| 48 |
| 1.5, 3 | 600, 800 | ssEPI, T2WI, T1WI, ADC | Asymptomatic high-risk with dense breast tissue with mammographically occult cancer + negative controls | 45 | 91 | |
| 343 |
| 3 | 1000 | rs-EPI MIP, rs-EPI fused to T1WI | Asymptomatic with history of breast cancer and no known active malignancy |
|
| |
| 113 |
| 3 | 850 | rs-EPI, ADC ‡ | Suspicious mammographic or ultrasound findings |
|
| |
| 106 |
| 3 | 850 | ss-EPI, ADC ‡ | Suspicious mammographic or ultrasound findings |
|
| |
| 166 |
| 3 | 800 | ss-EPI, TIRM, ADC ‡ | Dense breast and suspicious mammographic and/or MRI findings | 94 | 84 | |
| 378 |
| 1.5 | 1000 | ss-EPI, ADC | Known malignancy, suspicious mammographic or ultrasound findings and/or clinical symptoms, and intermediate- to high-risk screening | 93 § | 86 § | |
| 1130 |
| 3.0 | 1000 | ss-EPI, ADC ‡ | Contralateral breast of women with newly diagnosed unilateral breast cancer | 77.8 | 87.3 |
* Mean sensitivity and specificity for multiple readers was not reported in the original article and was calculated by the authors.
† Cancer prevalence calculations vary by study based on per a patient, b lesion, c breast, or d examination (as indicated) in order to match the performance metrics reported in the study.
‡ Quantitative ADC measurement was used as part of noncontrast imaging analysis.
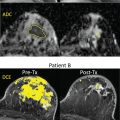
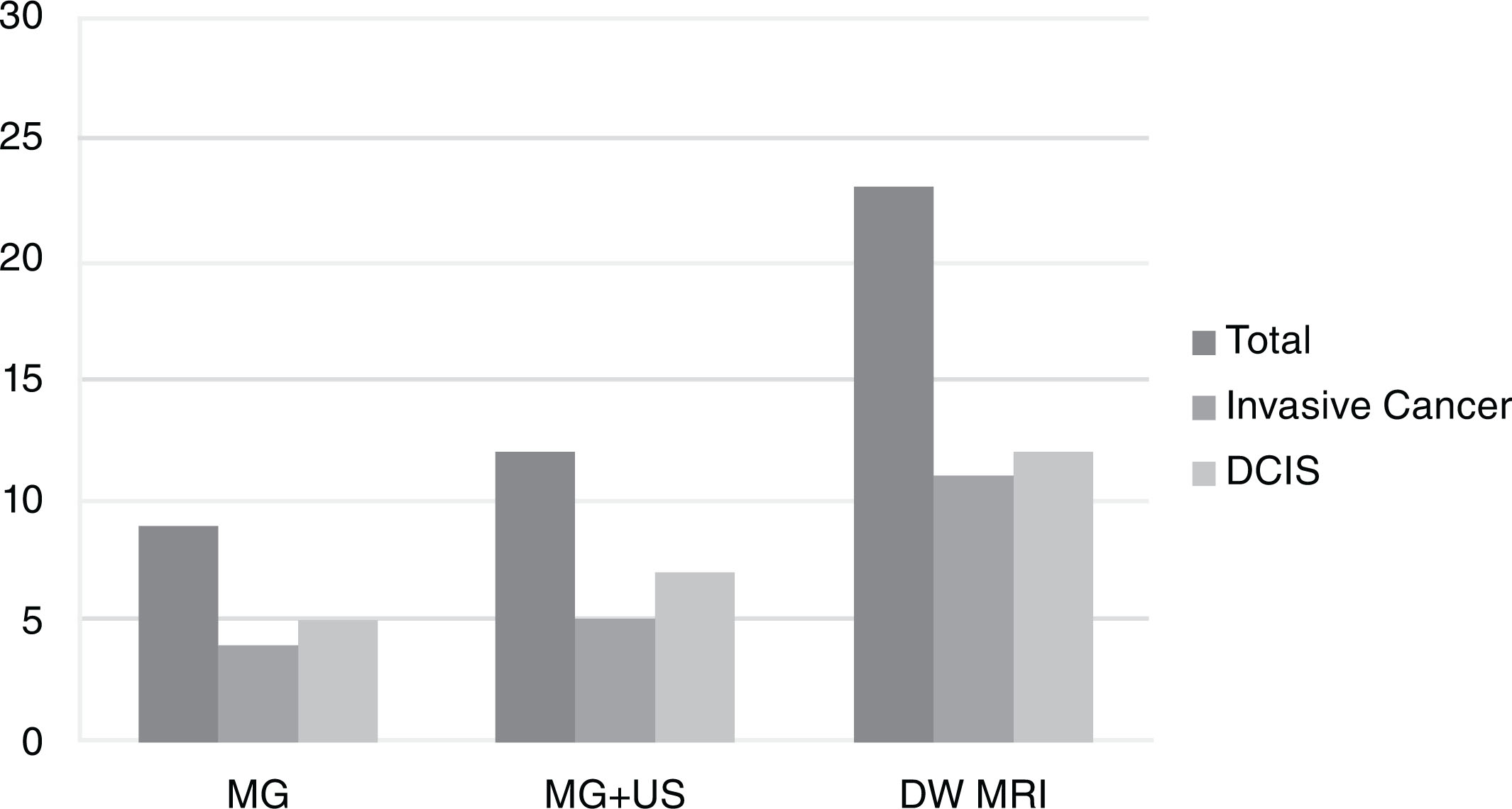
Some previous studies have reported on the performance of DW MRI versus other imaging modalities, including mammography, DCE MRI, abbreviated breast MRI, and ultrasound. Compared with mammography, DW MRI was found to be more sensitive (mean sensitivity across studies 78% vs. 59% for DW MRI vs. mammogram, respectively). Compared with DCE MRI, DW MRI was found to be less sensitive (mean sensitivity across studies 81% vs. 95% for DW MRI vs. DCE MRI, respectively). No studies directly compared blinded DW MRI performance with that of screening whole-breast ultrasonography; however, a nonblinded study of 60 mammographically occult cancers showed that more cancers were detectable on DW MRI (78%) compared with MRI-guided focused ultrasound (63%). In another study of 1146 women with newly diagnosed breast cancer, DW MRI of the contralateral breast showed higher sensitivity than mammography (77% and 30%, respectively) or combined mammography and ultrasound (40%) in detecting clinically occult cancer. The cancer detection rate (20 per 1000 examinations) and positive predictive value (42%) for biopsy recommendation of DW MRI was also higher compared with combined mammography and ultrasound (10 per 1000 examinations and 19%, respectively; Fig. 6.2 ) .
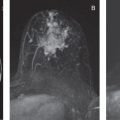
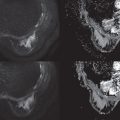
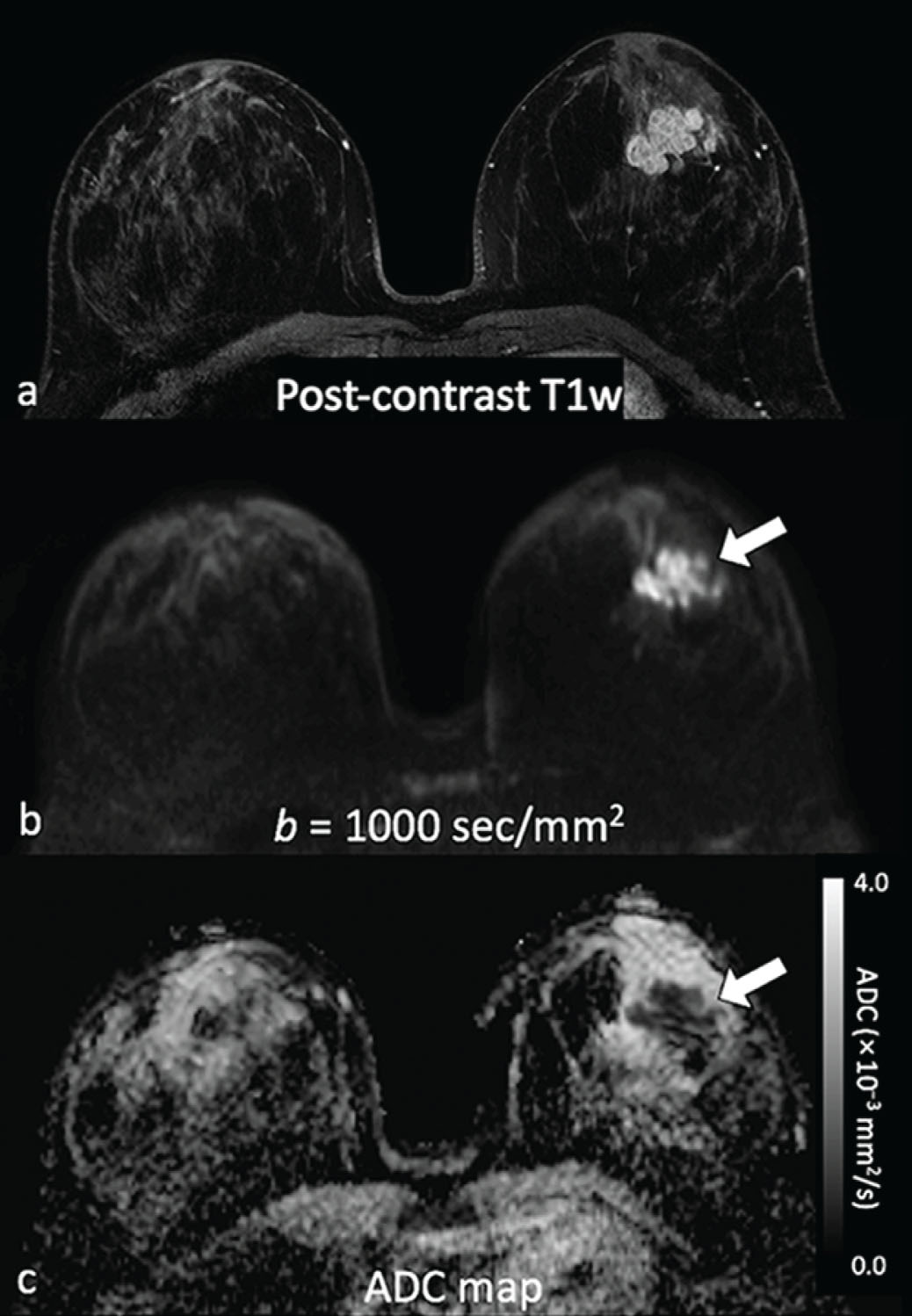
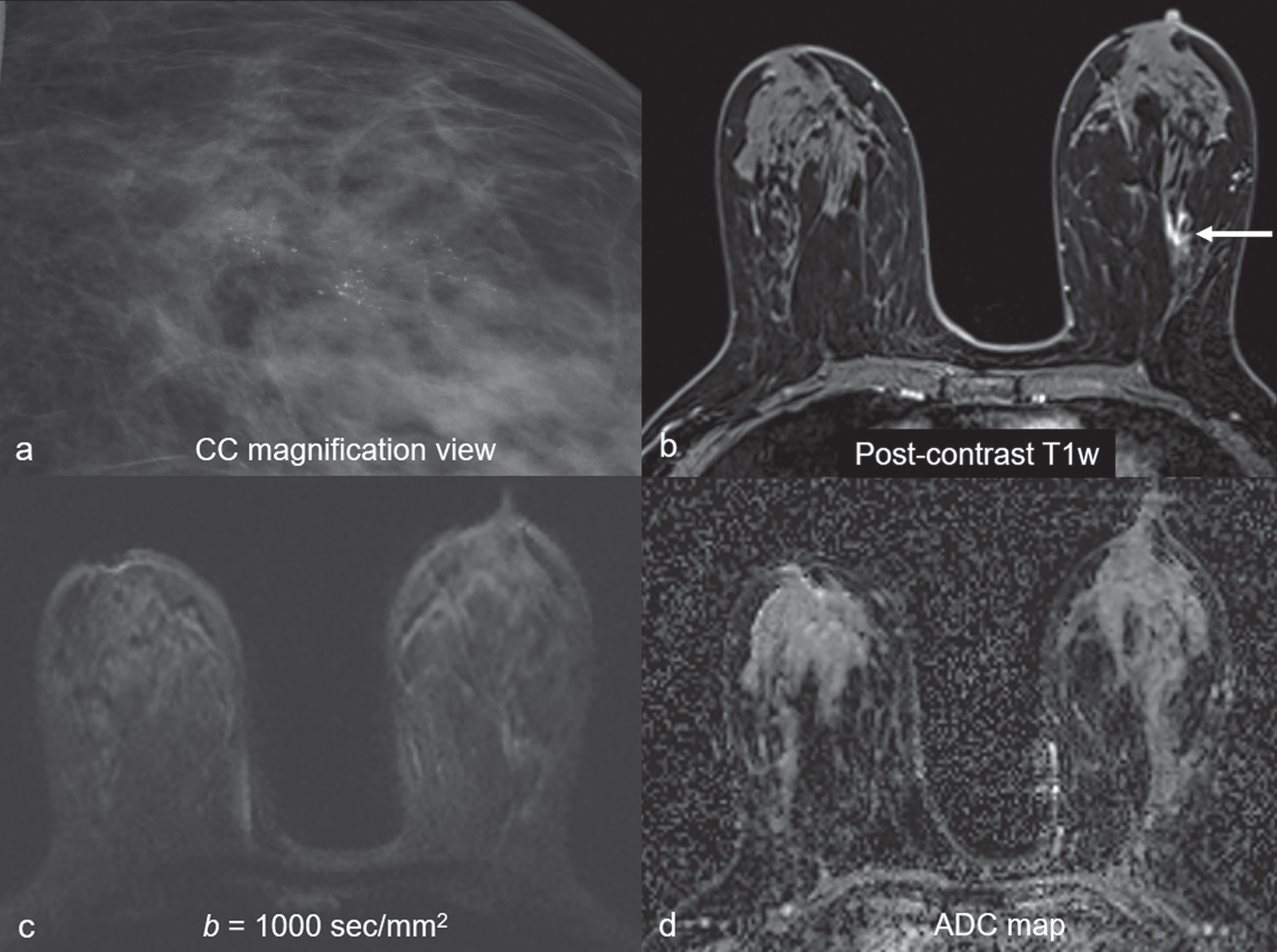
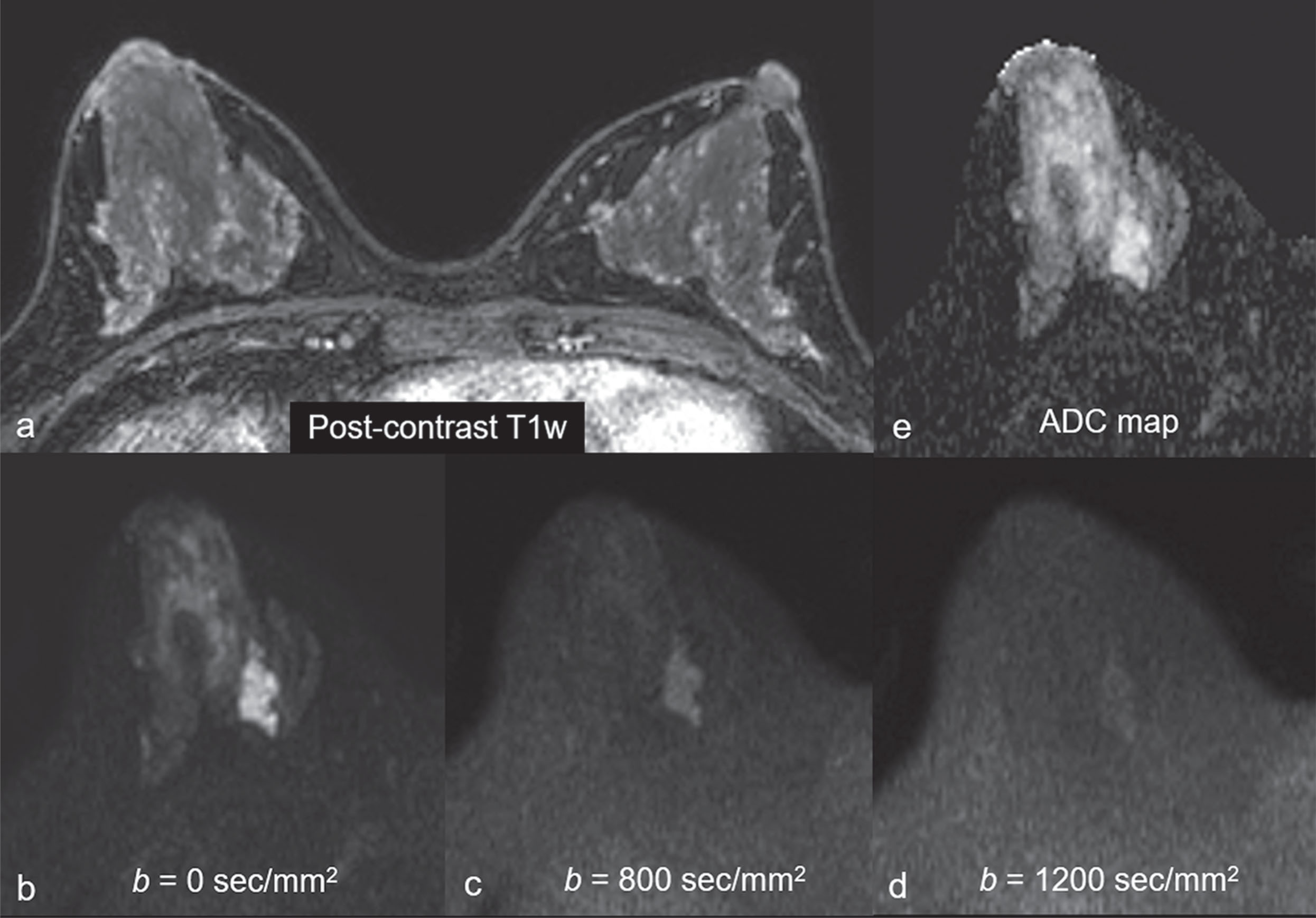
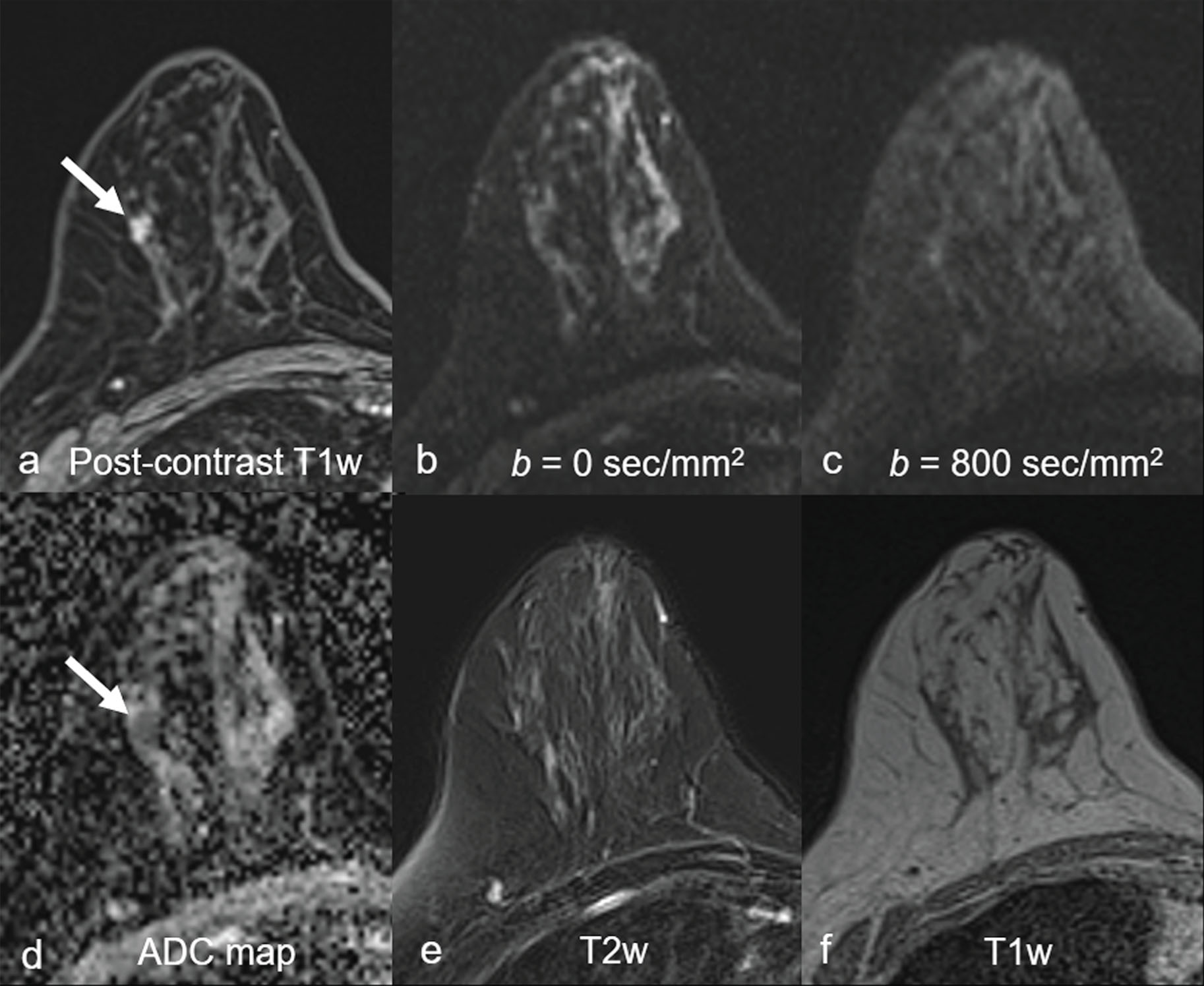
Common false-negative lesions in DW MRI include ductal carcinoma in situ (DCIS), mucinous carcinomas, and cancers presenting as nonmass enhancement and small masses. DCIS was more likely to be missed by DW MRI than invasive ductal carcinoma. DCIS commonly presents as a nonmass enhancement on DCE MRI with higher apparent diffusion coefficient (ADC) than invasive carcinomas, making it difficult to detect ( Fig. 6.3 ), with false-negative rates reported as high as 86% using this technique. Tumors with high liquid content, such as mucinous cancers and necrotic triple-negative cancers, can also exhibit a high ADC. Mucinous carcinoma was frequently missed on DW MRI ( Fig. 6.4 ), with a false-negative rate as high as 100%. Finally, small cancers (<10–12 mm) and invasive lobular cancer were also frequently missed ( Fig. 6.5 ). This was due to the low spatial resolution of conventional DW MRI techniques, which may lead to partial volume averaging, producing results that are not significantly better than the standard in-plane resolution and slice thickness (commonly 2 × 2 mm 2 and 3–5 mm, respectively). Expected false negative lesions on DW MRI also could include tumors with a low water content (e.g., low cellularity cancers with extensive desmoplastic stromal fibrosis).
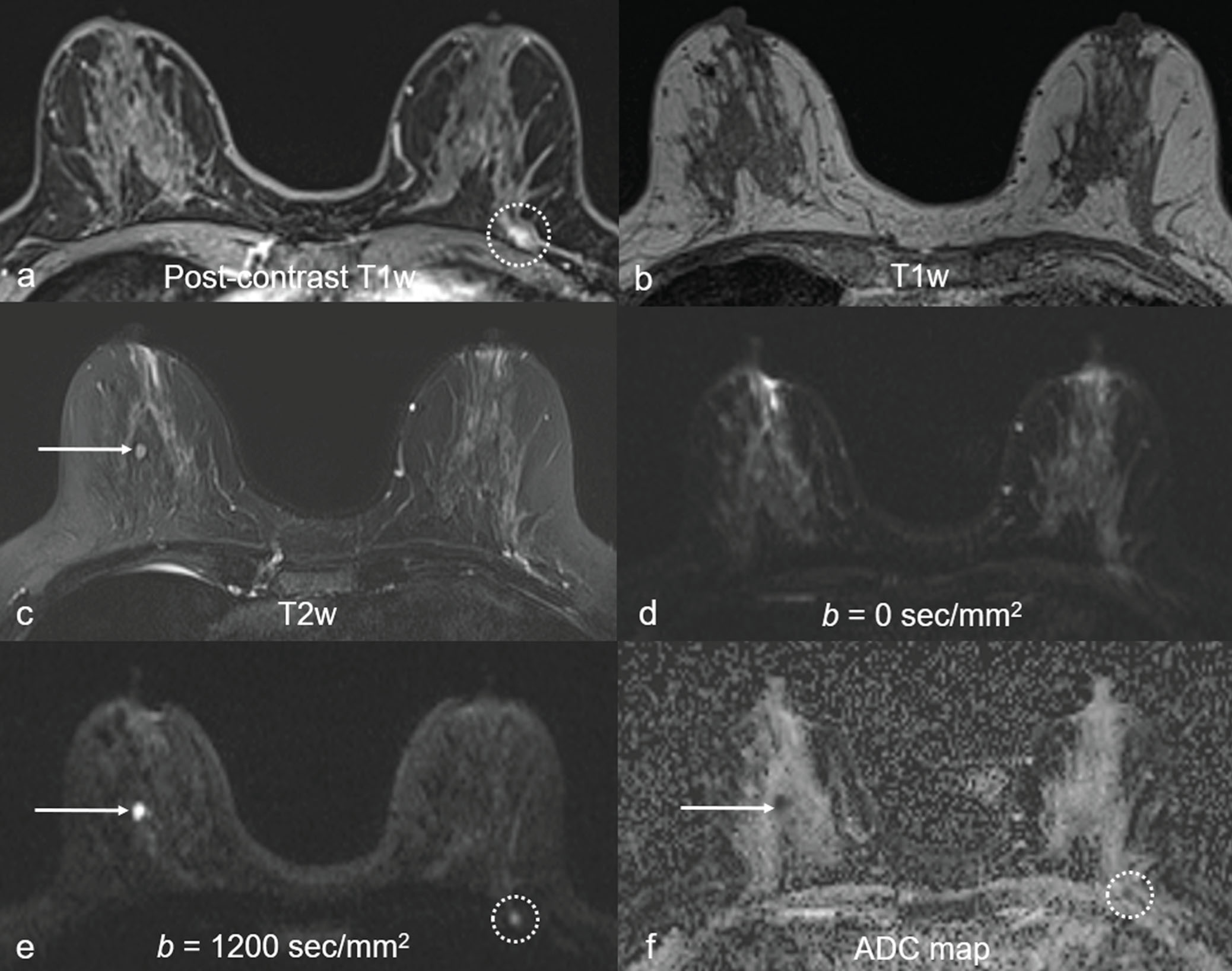
The notable false positives using DW MRI included complicated/proteinaceous cysts, fibroadenomas, and artifactual “lesions.” Complicated/proteinaceous cysts, which are known to exhibit restricted diffusion ( Fig. 6.6 ), represent the majority of DW MRI false positives in some settings. Similarly, fibroadenomas may be mistaken as a suspicious finding due to the wide range of possible ADC values. More than a third of fibroadenomas have ADCs in the same range as those of malignancies. Finally, false positives can be produced by artifactual signal, such as near the nipple-areolar complex, an area known to be prone to susceptibility-based distortion in DW MRI.
Technical Requirements as an Unenhanced Screening Modality
Expert consensus from the European Society of Breast Imaging (EUSOBI) recommended standardized parameters for high-quality breast DW MRI, which were extended specifically for the application of unenhanced breast cancer screening in a DW trial protocol ( Table 6.2 ).
| Minimum Requirement From EUSOBI | Acquisition Parameter From DWIST | |
|---|---|---|
| Study Purpose | Tumor Characterization | Cancer Detection |
| Equipment | ||
| Magnet field strength | ≥1.5 T | 3.0 T |
| Type of coil | Dedicated breast coil with ≥4 channels | 16 or 18 channels |
| Timing of acquisition | Before contrast injection, when possible | Before contrast injection |
| Acquisition Parameter | ||
| Type of sequence | EPI based | EPI based |
| Orientation | Axial | Axial |
| Field of view | Both breasts with or without covering the axillary region | Both breasts with covering the axillary region |
| In-plane resolution | ≤2×2 mm 2 | ≤1.3×1.3 mm 2 |
| Slice thickness | ≤4 mm | ≤3 mm |
| Number of b values | 2 | 3 |
| Lowest b value | 0 s/mm 2 (not exceeding 50 s/mm 2 ) | 0 s/mm 2 |
| High b value | 800 s/mm 2 | 800 s/mm 2 and additional acquisition of 1200 s/mm 2 |
| Fat saturation | SPAIR | SPAIR or STIR |
| Echo time (ms) | Minimum possible | Minimum possible |
| Repetition time (ms) | ≥3000 | ≥6000 |
| Acceleration factor | ≥2 | ≥2 |
| Postprocessing | Generation of ADC maps | Generation of computed multiple b values MIP series and ADC map |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree