Diffusion-weighted imaging (DWI) is feasible in the chest with currently available MR imaging scanners, although it is technically demanding. Although there is scarce clinical experience, the use of DWI has shown promising results in the characterization of pulmonary nodules, in lung cancer characterization and staging, and in the evaluation of mediastinal and pleural pathology. Ongoing research opens a door to noninvasive evaluation of heart fibers by means of diffusion-tensor imaging. Another area under investigation is the use of DWI of hyperpolarized gases as an early biomarker of pulmonary disease.
MR imaging of the chest has been traditionally challenging and difficult. Shortcomings for thoracic MR imaging are motion artifacts related to breathing and heart and vascular pulsation, susceptibility artifacts associated to air–tissue interfaces, and low proton density in both lungs creating low signal in all pulse sequences. Improvements in MR imaging systems, including more powerful gradients and phased-array coils, development of fast imaging techniques, such as echo-planar sequences (EPI), and application of parallel imaging, have made it possible to increase the clinical applications of thoracic MR imaging, although it is still far from being a first-line imaging test in pulmonary and mediastinal pathology. In a similar manner, cardiovascular MR imaging has developed in the last few years, with more clinical impact than pulmonary MR imaging.
In the era of functional imaging, diffusion-weighted imaging (DWI) has been proposed as a cancer biomarker. DWI allows the analysis of tissue characteristics based on the diffusivity of water molecules within the tissues. Although it was first used to detect acute cerebral ischemia, the use of DWI outside the brain has been possible in the last few years because of the previously mentioned technologic developments. Despite these advances, its use in the chest is still very challenging because of the high sensitivity of DWI to artifacts. Because of this, almost all the clinical studies of DWI in the chest have been performed in 1.5-T magnets. In addition, different methods of acquisition and quantification of DWI have been used. All of these facts have limited the clinical use of DWI in the thorax, with scarce clinical experience, mostly limited to detection and characterization of pulmonary nodules and mediastinal lymph nodes. Ongoing research with DWI in such areas as cardiac imaging and pulmonary ventilation makes DWI a potential clinical imaging tool in different areas and systems of the chest, which should be fully developed in the coming years.
This article clarifies which are the most appropriate sequences and technical adjustments for the different chest applications of DWI, including its use in 3-T magnets. Current realistic and potential clinical applications of DWI in the lungs, mediastinum, pleura, and heart are also analyzed.
Technical considerations
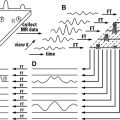
DWI is an MR imaging technique sensitive to the Brownian molecular motion of spins. The molecular motion (diffusion) is related to the thermal kinetic energy of the molecules, which is proportional to the temperature. In 1950, Hahn described that the presence of a magnetic field gradient during an MR imaging spin-echo (SE) experiment results in a signal attenuation because of the molecular diffusion of the spins. In 1965, Stejskal and Tanner proposed an MR imaging sequence to quantify the diffusion coefficient (D) in an MR imaging experiment. In their experiment, a pair of additional gradient pulses was inserted into a pulse sequence, the so-called “Stejskal–Tanner diffusion gradients.” This pulse sequence is still in use today in most DWI experiments, although some modifications have been proposed for moving organs, such as the heart. A new parameter (b, measured in seconds per square millimeter), is derived from the Stejskal–Tanner experiment, to control the image contrast in diffusion. This parameter is mainly controlled by the area under the two gradient lobes and the separation between them is used to weight diffusion. When higher b values are applied, the signal from the molecules that suffer a higher displacement is lost, with only the signal from those molecules with less displacement remaining.
The early DWI experiments were performed in stimulated-echo and SE pulse sequences. However, these pulse sequences required very long acquisition times of several minutes to acquire a single multislice data set, because they filled the required raw-data line by line. Therefore, these slow sequences were very prone to motion artifacts, which limited their usefulness in clinical applications, mainly in moving organs, such as the chest. Nowadays, the most extended pulse sequence for DWI is the single-shot (SS) SE EPI sequence. This sequence is relatively insensitive to macroscopic patient motion because of its very fast readout of the complete image data, within about 100 ms. It has become the standard technique for DWI and diffusion tensor imaging (DTI) not only for the brain but also for body applications.
Unfortunately, EPI images frequently suffer from gross geometric distortion in the presence of B0 inhomogeneities because of the accumulation of phase error during the long echo train length (ETL). This error is accumulated in phase acquisition direction, limiting the achievable resolution to maintain the geometric distortion under control. These distortions are particularly important in regions prone to magnetic susceptibility, such as bone–soft tissue interfaces or those structures in contact with air-filled spaces, as occurs in the chest.
To avoid the artifacts associated to SS EPI acquisitions, different strategies have been proposed. The most sensible one is to segment the ETL of the EPI acquisition in different shots, reducing the phase error accumulated during different readouts. Although this approach has fewer geometric artifacts, the acquisition time increases proportionally to the number of EPI shots. Besides, these sequences are more prone to motion artifacts, making it necessary to apply motion correction techniques, such as navigation echoes. A special application of the multishot EPI readout is the “periodically rotated overlapping parallel lines with enhanced reconstruction” acquisition. This sequence organizes the segmented (PROPELLER) acquisition in a radial way around the center of k-space. This approach has the advantage that the results are less sensible to motion artifacts. A different strategy to reduce the ETL without increasing the acquisition time is to apply parallel imaging, where the phase encoding lines that are not acquired are recovered using the sensitivity profile of phased array coils.
Recent innovations in hardware and acquisition techniques have substantially improved the suitability of EPI for chest DWI. Improved gradient systems with reduced eddy-current effects have allowed faster EPI readout, which can decrease geometric distortions. Moreover, new gradient technology, reaching gradient strength of 80 mT/m, makes it feasible to acquire DWI with a b value up to 1000 s/mm 2 and an echo time (TE) under 45 milliseconds, with an acquisition matrix of 128 × 128.
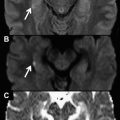
Another important aspect related to the combination of EPI readout with DWI is the intensity of fat signal for very high b values. Fat signal has a very low diffusion coefficient, which makes it very relevant for high b values. However, the difference in precession frequency between the water and the fat produces a water-fat shift of several voxels in the phase encoding direction of the EPI readout. Because of both factors, the fat signal usually overlaps on the studied anatomy making it mandatory to apply fat suppression techniques for more accurate apparent diffusion coefficient (ADC) estimation. When studying the chest, the short tau inversion recovery (STIR) approach has been most commonly used as a fat suppression technique in such sequences as DWI with background suppression (DWIBS). The main problem of sequences using STIR is the low signal to noise ratio (SNR) caused by water signal reduction after the inversion pulse. To overcome this problem, different spectral fat suppression techniques, such us spectral presaturation inversion recovery (SPIR) and spectral selection attenuated inversion recovery, have been proposed, because of their superior SNR to acquisitions using STIR ( Fig. 1 ).
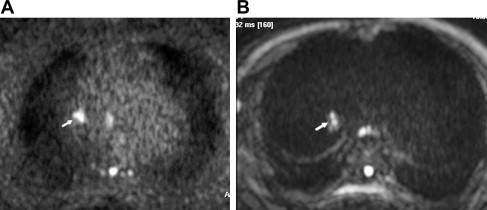
To solve the lack of spatial resolution of DWI sequences, the use of higher field magnets as 3 T has been proposed for body applications. For example, a signal improvement of 50% has been reported in kidney studies when comparing 3 T with 1.5 T within the same acquisition time. The increase of signal of 3-T magnets may be used to obtain higher resolution or to reduce scan time. The acquisition problems inherent to DWI increase in 3-T magnets, because of higher magnetic field variation and susceptibility artifacts, which produce image distortion, and SAR limitations, which make fat suppression diffcult. These limitations can be overcome using appropriately higher strength of the gradient systems of 3-T scanners in combination with parallel imaging and advanced fat suppression sequences. In our experience, all these tools make it feasible to acquire thoracic DWI studies in 3-T systems ( Fig. 2 ). Moreover, Gill and colleagues recently reported the first clinical series of DWI performed on a 3-T magnet, with satisfactory evaluation of 57 patients with malignant pleural mesothelioma (MPM), although ADC quantification could not be obtained in seven patients because of image distortion.
The authors’ standard sequences for DWI of the chest at 1.5- and 3-T magnets are detailed in Table 1 ; their sequence recommendation when possible is as follows:
- •
SS SE EPI
- •
Phased array surface coil
- •
b-values: several values between 0 and 100 s/mm 2 until 1000 s/mm 2
- •
Field of view: 320–400
- •
Parallel Imaging acceleration factor of 2
- •
Pixel resolution 2.5 × 2.5 × 7 mm 3
- •
Spectral fat suppression
- •
Number of slices, 24
- •
TR: 5000 ms
- •
TE: 53 milliseconds (shortest)
- •
Respiratory triggered
- •
Three orthogonal motion probing gradients.
| Sequence Type/Parallel Acceleration Factor | B values (s/mm 2 ) | TR/TE (ms) | Resolution (mm 3 ) | Synchronization | Fat Suppression Technique | Image Evaluation | |
|---|---|---|---|---|---|---|---|
| IVIM 3-T | SS EPI/factor 2 | 0, 10, 20, 30, 50, 100, 150, 300, 450, 600, 750, 1000 | 5000/55 | 2.5 × 2.5 × 7 | Respiratory triggered | Spectral fat suppression | IVIM model |
| DWIBS 3-T | SS EPI/factor 2 | 0–1000 | 5000/55 | 2.5 × 2.5 × 7 | Respiratory triggered | STIR (inversion time 260 ms) | ADC |
| IVIM 1.5-T | SS EPI/factor 2 | 0, 50, 100, 150, 400, 600, 1000 | 1400/100 | 3 × 3 × 7 | Respiratory triggered | Spectral fat suppression | IVIM model |
| DWIBS 1.5-T | SS EPI/factor 2 | 0–1000 | 632/60 | 3 × 3 × 7 | Respiratory triggered | STIR (inversion time 160 ms) | ADC |
ADC Quantification
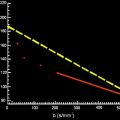
From the Stejskal and Tanner acquisition sequence, it can be derived that the signal attenuation caused by DWI has an exponential behavior, modulated by the control sequence parameter, b value, and the diffusion properties of the tissue. In the presence of single water compartment the diffusion signal can be expressed as:
S ( b ) = S 0 e − b D ,
D = 1 b max − b min ln ( S ( b min ) S ( b max ) ) .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree