Chapter Outline
The vermiform appendix is the smallest and functionally most irrelevant segment of the gastrointestinal (GI) tract, yet diseases of the appendix are among the most common surgical emergencies in the western world. The appendix arises from the posteromedial aspect of the cecum at the junction of the three taeniae coli. Although the appendix has the same mural layers as those of the remainder of the gut, it is distinguished by extremely rich lymphoid tissue in the mucosa and submucosa. This forms an entire layer of germinal follicles and lymphoid pulp in the young. With age, this lymphoid tissue underlying the mucosal epithelium and glands undergoes progressive atrophy. The distal portion of the appendix sometimes undergoes fibrous obliteration in older adults.
Embryology
The vermiform appendix and cecum are intestinal derivations of the midgut. During the sixth week of gestational life, the cecum begins to develop as a small bulge in the midgut at the level of the ileocolic junction. This diverticular structure continues to enlarge and assumes a conical shape, with its apex corresponding to the primitive appendix. The initial cecal and appendiceal structures have been referred to as the “bud of the cecum.” The midgut undergoes physiologic umbilical herniation at approximately gestational week 6 and subsequently begins to relocate into the embryonal body cavity at approximately gestational week 10. This process is accompanied by counterclockwise rotation of the midgut, which results in positioning of the cecal complex in the right lower quadrant ( Fig. 56-1 ). As the colon grows, it moves caudad toward the right iliac fossa, a process known as descensus. Because of differential growth rates during this time, the development of the primitive appendix is slower than that of the rest of the cecum. This differential growth was theorized by Broman to be caused by a mucosal fold in the distal cecum that prevents accumulation of meconium. Broman hypothesized that colonic growth is stimulated by intraluminal meconium accumulation. Because this mucosal fold prevents complete filling of the distal cecum and appendix with meconium, growth is retarded in these segments. The appendix subsequently lengthens rapidly but fails to grow in thickness, soon assuming the configuration of a vermiform process.
By birth, the appendix has lengthened and shows a more abrupt transition zone at the junction with the cecum. At this time, the appendix is still attached to the cecal apex. The fully developed adult appendix is uniformly narrow and arises from the left posterior wall of the cecum, rather than the cecal apex, 2.5 to 3.5 cm below the ileocecal valve. The process of migration of the root of the appendix from the cecal tip toward the left, on the same side as the ileocecal valve, is probably related to the upright posture of the child. The right and ventral walls of the cecum grow and distend at the expense of the left and posterior walls. The fixation at the ileocecal junction and weight of the column of feces in the ascending colon explain this additional asymmetric development.
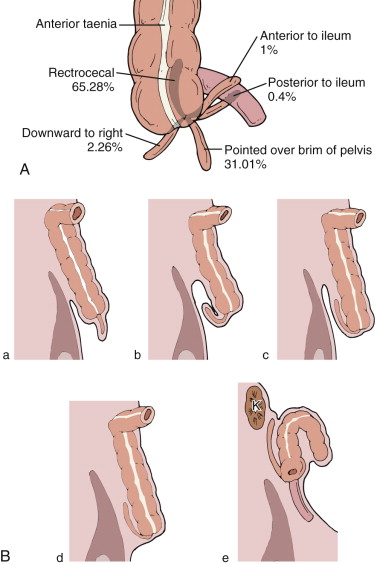
In adults, the root of the appendix can be located anywhere along the medial-posterior wall of the cecum, between the ileocecal valve and the cecal tip. In addition, there is great variation in the position of the appendix in relation to the cecum and ascending colon. More than 60% of appendices are retrocecal or retrocolic, with the remainder in a more inferior pelvic location. A fully developed appendix varies in size and thickness, with an average length of 8 to 10 cm (range, 4-25 cm; Fig. 56-2 ). The location of the appendix in the peritoneal cavity depends on its length and relationship to the cecum and the extreme variations in the mobility and location of the ascending colon and cecum ( Fig. 56-3 ).
Examples of arrest in later phases of development of the appendix are common and can be recognized during barium enema examinations. An arrest in the fetal phase of development is unusual ( Fig. 56-4 ). A conic configuration of the lower cecal segment signifies total absence or hypoplasia of the appendix. Agenesis of the appendix is rare; the reported incidence is about 1 in 100,000 individuals.
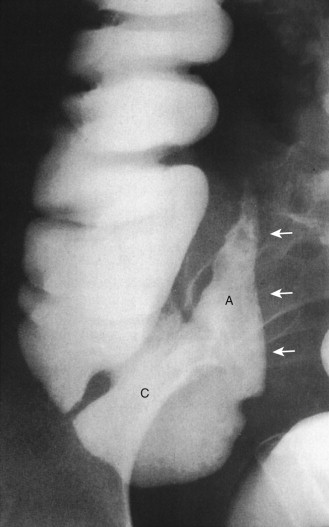
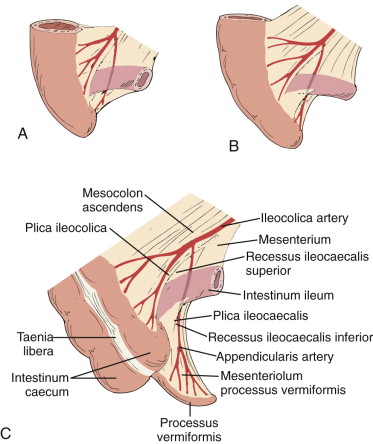
The ileocolic artery, a branch of the superior mesenteric artery, supplies the appendix, cecum, and ileum via five branches: (1) several ileal rami; (2) anterior cecal artery; (3) posterior cecal artery; (4) colic ramus; and (5) appendicular artery, which runs through the mesenteriolum to supply the vermiform appendix. Because there are no arterial arcades in the mesenteriolum, the appendicular artery is a terminal artery, predisposing the appendix to ischemia when there is vascular insult. The appendicular artery origin is variable, with the following frequencies: iliac ramus (35%), ileocolic artery (28%), anterior cecal artery (20%), posterior cecal artery (12%), ileocecal artery (3%), and ascending colon ramus (2%). The veins accompany the arteries.
Lymphatic tissue begins to develop in the appendix during the 14th and 15th weeks of gestation. Initial accumulations of lymphatic cells occur directly below the epithelium, but some lymphocytes eventually penetrate the epithelial layer of the appendix. Lymphatic drainage of the appendix is via ileocolic lymph nodes located along the superior mesenteric artery and via celiac nodes into the cisterna chyli.
Unlike the cecal wall, which is composed of a diagonal rhomboid mesh of collagen fibers and allows it to accommodate luminal expansion, the appendiceal wall is composed of horizontal collagen fibers that tolerate only minimal increases in luminal diameter. Although the appendix secretes 2 to 3 mL of mucus daily, its average luminal capacity is only approximately 1 mL. This, in conjunction with the appendix’s solitary blood supply, explains its propensity to become ischemic rapidly and perforate after luminal obstruction.
Acute Appendicitis
Epidemiology
Appendicitis was first described and reported by Reginald H. Fitz at the 1886 meeting of the Association of American Physicians. Appendicitis is the most frequent cause of acute abdominal pain requiring surgical intervention in the western world and is the most common abdominal operation performed annually in the United States on an emergency basis.
Appendicitis is rare in infants but becomes increasingly common in childhood. The highest incidence of appendicitis is in individuals aged 10 to 19 years (15.3/10,000 population/year). Across all age groups, the annual rate is approximately 9.4/10,000. The lifetime cumulative incidence is approximately 9%. There is a slight male predominance, with a ratio of 1.3 : 1. Mortality and morbidity rates for removal of a normal appendix are 0.14% and 4.6%, respectively, but increase to 0.24% and 6.1% for acute appendicitis and to 1.7% and 19% for perforated appendicitis, respectively. These mortality rates are far better than the 50% mortality seen in the preantibiotic era.
Appendiceal perforation occurs in 16% to 39% of patients with a median reported incidence of 20%. Risk factors for perforation include very young or old age, Asian or Hispanic ethnicity, and lower socioeconomic status. Although some early studies found a linear correlation between perforation rates and diagnostic accuracy, more recent studies have cast doubt on any relationship. A study of 1486 appendectomy cases found an overall perforation rate of 19%, with higher rates in the very young and older adults. Vorhes noted that patients older than 55 years underwent laparotomy on average 2 days later than those younger than 55 years. A large retrospective study of children (≤18 years) showed that the rate of appendiceal perforation was markedly higher for children younger than 5 years old (71.9% vs. 21.7% for children > 5 years) and that the risk for perforation was inversely proportional to the patient’s age.
Acute appendicitis is the most common nonobstetric surgical emergency in pregnant patients, occurring in approximately 1 in 766 pregnancies. Complications include premature labor and fetal and maternal death, especially when perforation occurs. The fetal loss rate for nonruptured appendicitis is approximately 2%, compared with more than 30% in patients with ruptured appendicitis.
Causes
Acute appendicitis occurs after luminal obstruction. Causes of obstruction include fecalith, lymphoid hyperplasia, primary tumor (e.g., carcinoid, adenocarcinoma, lymphoma, Kaposi’s sarcoma) or metastasis, parasitic infection, foreign body, stricture, Crohn’s disease, and adhesion ( Fig. 56-5 ). Prolonged retention of barium is no longer considered a risk factor for acute appendicitis.
Of the possible causes of luminal obstruction, fecaliths are the most common, occurring in 11% to 52% of patients with acute appendicitis. Fecalith formation results from inspissation of fecal material and inorganic salts within the appendiceal lumen. As they enlarge, these concretions can obstruct the appendix. Low-fiber diets contribute to low-residue stool, which has a propensity to become impacted in the appendiceal lumen. True appendiceal calculi (hard, noncrushable calcified stones) are less common than fecaliths but, when present, they have a higher association with appendiceal perforation and periappendiceal abscess formation.
Hygiene improvements of the modern world have greatly reduced the exposure of infants and children to enteric organisms. Consequently, when infections do occur, they can elicit an exaggerated lymphoid hyperplasia that may block the appendix or devitalize the appendiceal mucosa, allowing bacterial invasion.
Pathophysiology
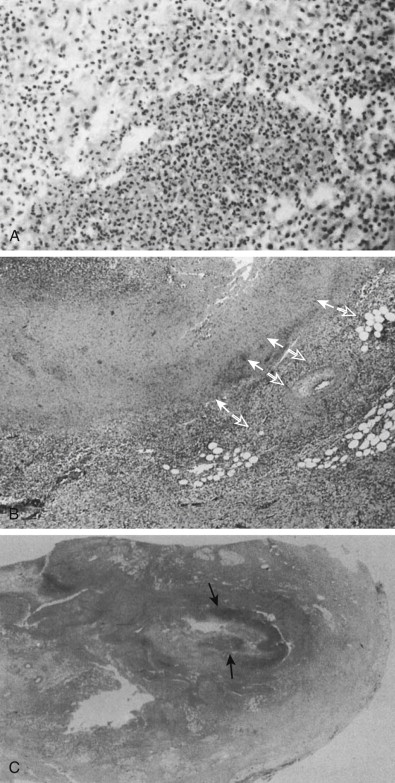
Continued secretion of mucus into the appendix after luminal obstruction results in distention and concurrent elevation of luminal pressures. When the luminal pressure exceeds the capillary perfusion pressure, lymphatic and venous drainage are impaired and arterial compromise and tissue ischemia result. Breakdown of the epithelial mucosal barrier occurs, and luminal bacteria multiply and invade the appendiceal wall, causing transmural inflammation and gangrenous appendicitis ( Fig. 56-6 ). Continued tissue ischemia can result in appendiceal infarction and perforation. Perforation can cause a localized or generalized peritonitis as inflammation extends through the serosa to the parietal peritoneum and adjacent organs (cecum, terminal ileum, pelvic viscera). Although periappendiceal abscess or phlegmon is usually walled off by the adjacent greater omentum or small bowel, some cases will spread through the peritoneum to the paracolic gutters and subphrenic spaces, sigmoid colon, bladder, ovaries, or vagina. Infected thrombus may form in the portal vein, causing pylephlebitis, although this is unusual with modern antibiotics.
Clinical and Radiologic Assessment
Clinical assessment remains an essential and critical part of the initial evaluation of patients with suspected acute appendicitis. The physician’s goal is to confirm or exclude the diagnosis of acute appendicitis expeditiously while minimizing diagnostic delays, negative appendectomy (i.e., surgery for suspected appendicitis yields a normal appendix) and appendiceal perforation rates, and hospital costs. More recently, reducing or eliminating ionizing radiation dose to the patient has become a major consideration driving the diagnostic approach, particularly in children and pregnant women. However, despite continued advances in clinical medicine, the diagnosis of acute appendicitis can sometimes remain elusive.
Although diagnostic accuracy rates for appendicitis in the United States had improved from 86% to 92% in male patients and from 74% to 83% in female patients between 1970 and 1984, one study suggested that imaging has helped improve accuracy. Perforation and false-negative appendectomy rates have stabilized in recent decades. Cases of “missed appendicitis” typically reflect the frequent diagnostic difficulty of differentiating appendicitis from other causes of abdominal pain.
Historically, negative appendectomy rates of as high as 15% to 23% had been acceptable, with rates as high as 45% in patients with an atypical presentation or in women of childbearing age. Negative laparotomy rates of 10% to 15% have now been considered acceptable, although that number continues to trend downward as imaging improves. Negative laparotomy rates remain higher in women of childbearing age. Diagnostic accuracy can be improved with inpatient observation, subsequently leading to a decreased negative appendectomy rate without a concurrent increase in appendiceal perforation rates.
A large retrospective study found that risk factors for negative appendectomy included very young or advanced age, female gender, and presence of comorbid illness. In that study, the highest rate of negative appendectomy was in women older than 70 years. Negative appendectomy (compared with positive appendectomy) was associated with a significantly longer length of stay (5.8 vs. 3.6 days), case-fatality rate (1.5% vs. 0.2%), and infectious complication rate (2.6% vs. 1.8%).
Radiologic imaging has become an important adjunct to clinical evaluation. Accurate identification of an inflamed or normal appendix can greatly facilitate appropriate treatment. Imaging can also identify alternate diagnoses in patients with clinically suspected acute appendicitis.
Despite advances in radiologic imaging, controversy still exists in the literature about whether the increased availability of imaging and laparoscopy has decreased misdiagnoses and negative laparotomy rates. For example, Flum and associates performed a retrospective study of 63,707 appendectomy patients from 1987 to 1998. They found that, contrary to expectation, the use of computed tomography (CT), ultrasonography (US), and laparoscopy did not change the negative appendectomy or appendiceal perforation rates.
In contrast, many subsequent reports have described reductions in the negative appendectomy rate associated with increased use of imaging. Jones and co-workers found a decrease in the negative appendectomy rate (from 17%-2%) coinciding with increased CT use from 2000 to 2002. The rate of appendiceal perforation also decreased (from 25%-9%) during the same interval. Rao and colleagues found reductions in the negative appendectomy rate (from 20%-7%) and perforation rate (from 22%-14%) with increased use of CT. At Duke University, a 10-year retrospective study published in 2010 found that an increase in preoperative CT use from 18.5% to 93.2% resulted in a reduction of the negative appendectomy rate among 18- to 45-year-old women from 42.9% to 7.1%. A study by Bendeck and associates showed a lower negative appendectomy rate for women who underwent preoperative CT or US imaging compared with women without preoperative imaging (7% vs. 28%, respectively).
In a large study of almost 20,000 patients, Drake and co-workers found a negative appendectomy rate of 4.5% in patients who underwent imaging versus 15.4% in patients with only clinical assessment. Despite imaging, the negative appendectomy remained higher for women compared with men (6.9% vs. 3%).
Findings
Clinical Findings
Most patients with acute appendicitis present with abdominal pain, although the classic presentation sequence of poorly localized periumbilical pain followed by nausea and vomiting and later migration of the pain to the right lower quadrant occurs in only half to two thirds of all patients. The location of abdominal pain varies and depends on both the position of the inflamed appendix and stage of appendiceal inflammation. With initial distention and increased intraluminal pressure in the obstructed appendix, patients typically perceive visceral epigastric or periumbilical pain. During this time, the disease is usually confined to the appendix. When the inflamed serosa of the progressively inflamed appendix comes into contact with the parietal peritoneum, somatic pain is perceived, with the classic description of pain that shifts to the right lower quadrant. In patients with a retrocecal appendix, the pain may be referred to the right flank, costovertebral angle or, in males, the right testis. Patients with a pelvic or retroileal appendix may experience pain in the pelvis, rectum, adnexa or, less commonly, left lower quadrant. Nausea, vomiting, and anorexia are variably present, occurring in more than 50% of all cases.
Signs and symptoms vary with the inflammatory stage of the appendicitis. Abdominal tenderness is the most common physical finding, occurring in more than 95% of patients. Although the classic teaching is that patients with appendicitis present with localized tenderness at or near McBurney’s point (positioned 1.5 cm superior and medial to the anterior superior iliac spine and parallel to a plane drawn from the anterior superior iliac spine to the umbilicus), the point of tenderness will vary, depending on the position of the inflamed appendix. In one series of 275 double-contrast barium enemas, the appendiceal location was within 5 cm of McBurney’s point in only 35% of patients and was farther than 10 cm in 15% of cases. Usually, the appendix is located inferior and medial to McBurney’s point. Patients may also present with Rovsing’s sign (pain referred to the area of maximal tenderness during palpation or percussion of the left lower quadrant), positive psoas sign (right lower quadrant pain with extension of the right hip), or obturator sign (right lower quadrant pain with flexion and internal rotation of the right hip). Voluntary muscle guarding in the right lower quadrant is common and typically precedes localized rebound tenderness. Bowel sounds vary but are usually diminished or absent with advanced appendiceal inflammation or perforation.
Most patients with acute appendicitis (70%-90%) present with a white blood cell (WBC) count greater than 10,000/mL and neutrophilia more than 75%. Serial WBC counts may be helpful in the diagnosis because it has been shown that patients tend to have an increased WBC count 4 to 8 hours after admission unless the appendix is perforated, in which case the WBC count typically decreases. Because a minority of patients with acute appendicitis have a normal WBC count, a normal value is not sufficient to exclude the diagnosis.
Urinalysis is positive in 19% to 40% of patients with appendicitis; abnormalities include bacteriuria, mild pyuria, and hematuria. Abnormal urinalysis results are more commonly observed in women with appendicitis than in men.
Elevation of the C-reactive protein (CRP) level more than 0.8 mg/dL has sensitivities of 46% to 75% and specificities of 56% to 82% in acute appendicitis, and elevation is more common when symptoms are present for more than 12 hours. The diagnostic sensitivity for acute appendicitis is improved to 97% to 100% when an elevated CRP level, elevated WBC count, and neutrophilia more than 75% are all present.
Imaging Findings
Plain Radiographs.
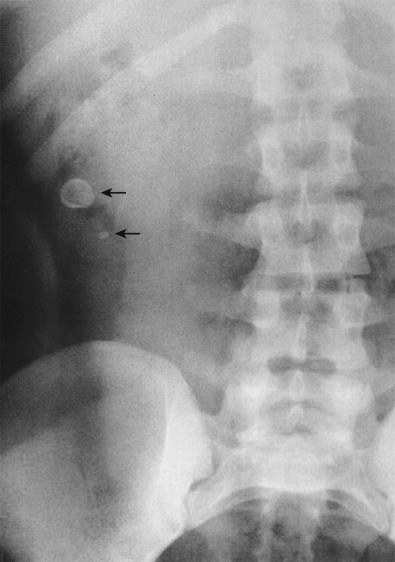
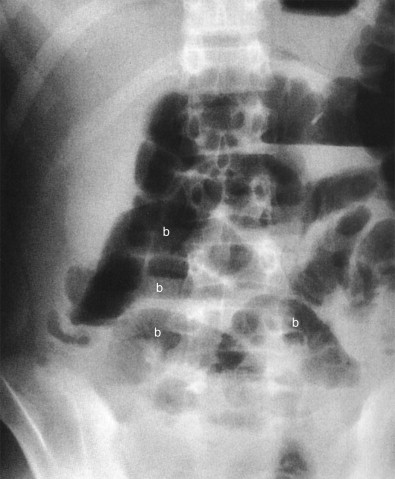
The most specific plain film sign is the presence of an appendicolith ( Fig. 56-7 ). However, appendicoliths are only found by radiographs in 10% of patients with acute appendicitis. When present on radiographs, the reported incidence of perforation is almost 50%. Appendicoliths are usually 0.5 to 2 cm in diameter and have a round or oval configuration and laminated rim. The calcified rim assists in differentiating them from bone islands, ureteral stones, and phleboliths. They may be obscured by the bone structures of the pelvis or may be ectopically located in the right upper abdomen in cases of retrocolic appendicitis. Appendicoliths are usually solitary, but two or three adjacent small calcifications are not unusual. Appendicoliths may be detected in asymptomatic individuals and, without associated clinical findings, are not indicative of appendicitis.
Air in the appendix, particularly in the retrocecal location, is a normal finding. Extraluminal bubbles of air associated with an ill-defined soft tissue mass indicate an abscess ( Fig. 56-8 ). Sometimes, the inflammatory process in the right lower quadrant induces a severe localized ileus, with dilation and air-fluid levels in the ileal loops and cecum. When severe, this process can mimic the appearance of a mechanical distal small bowel obstruction ( Fig. 56-9 ). Dilation of the transverse colon in association with a gasless cecum and ascending colon may result from ileus of the transverse colon and spasm of the ascending colon. Free air in the peritoneal cavity is rare because the base of the appendix is usually occluded when perforation occurs.
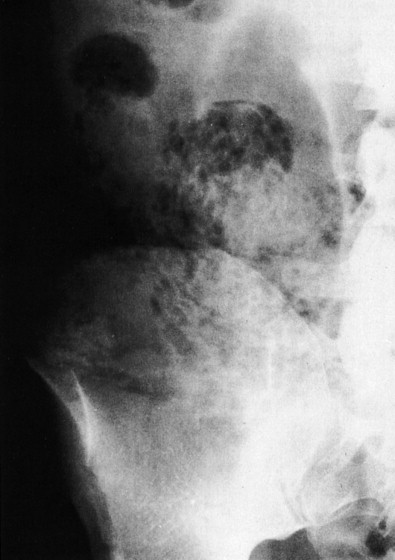
Other findings such as partial loss of the right psoas shadow and a lumbar scoliosis concave to the right are common, albeit nonspecific. Appendicitis may also cause a distal small bowel obstruction, particularly when perforated.
Plain film radiography is typically inadequate for the diagnosis of appendicitis. Ahn and colleagues retrospectively reviewed the records of 871 adult patients with nontraumatic acute abdominal pain and found that plain radiographs were normal in 23%, nonspecific in 68%, and abnormal in only 10%. The diagnostic sensitivity for appendicitis, pyelonephritis, pancreatitis, and diverticulitis was 0% in the study.
Barium Enema.
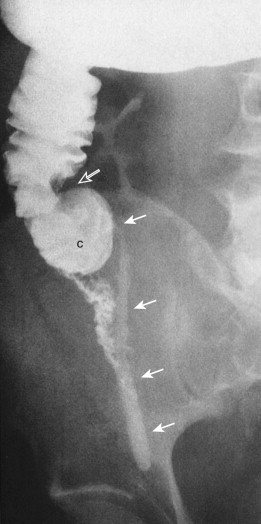
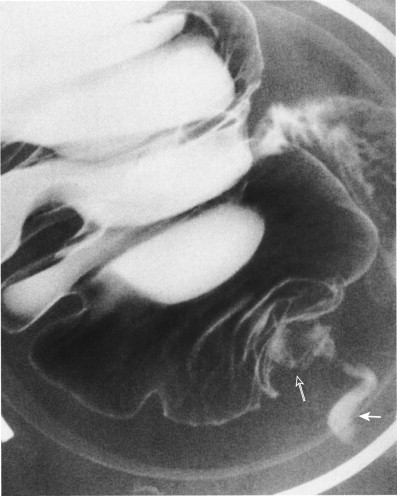
Before the 1980s, the barium enema was the primary radiologic test used in the diagnosis of appendicitis. This examination can be performed quickly and safely with the single-column technique. Because appendicitis results from luminal obstruction, complete filling of a normal appendix effectively excludes the diagnosis ( Fig. 56-10A ). Nonfilling or incomplete filling of the appendix in the presence of mass effect (on the caput cecum and adjacent distal ileum indicates inflammation Fig. 56-11 ; Fig. 56-10B ). Studies have reported diagnostic accuracy as high as 91.5%.
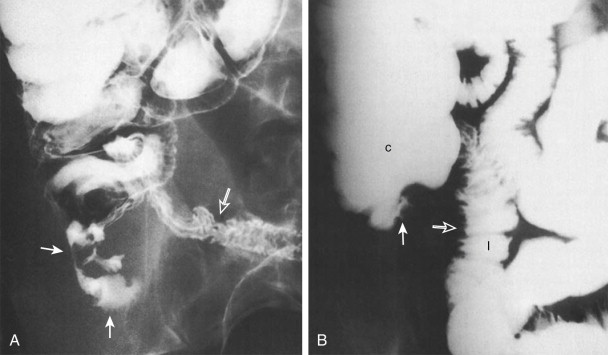
A barium enema study may also detect other pathologies of the small and large bowel that can mimic the clinical presentation of acute appendicitis. These include neoplasm, Crohn’s disease, ileal diverticulitis, and cecal diverticulitis.
Barium enema is not without major drawbacks. Nonfilling of the appendix can be seen in 15% to 20% of normal patients, and it may be difficult to differentiate a partially filled from a completely filled appendix. Also, a barium enema study provides only inferential information about extracolonic disease and cannot evaluate the nature of appendiceal phlegmons and abscesses. The appendix can also intussuscept ( Fig. 56-12 ) into the cecum, producing a cecal defect unrelated to appendicitis.
Computed Tomography.
Helical CT imaging has proven to be a highly effective and accurate means of diagnosing acute appendicitis, with reported sensitivities of 90% to 100%, specificities of 91% to 99%, accuracies of 94% to 98%, positive predictive values of 92% to 98%, and negative predictive values of 95% to 100%. In addition to visualization of the appendix, CT can detect complications of appendiceal perforation, such as abscess or phlegmon ( Fig. 56-13 ). Critically, CT can help diagnose many alternate conditions that might mimic the clinical presentation of acute appendicitis.
The goal of CT investigation in patients with right lower quadrant pain is to identify the normal or abnormal appendix. Thin-section (slice thickness ≤5 mm) imaging is now standard, providing superior sensitivity compared with older techniques. With the advent of multidetector scanners such as 16- and 64-slice multidetector CT (MDCT), high-quality multiplanar reformatted (MPR) images are now possible. Adding coronal reconstructions, for example, can improve appendix visualization and diagnostic confidence.
Radiologists are able to identify a normal appendix in most patients with abdominal pain who undergo abdominal CT (67%-100%), with a trend toward better visualization with thinner section imaging. Appendix identification is more difficult in patients with a paucity of retroperitoneal fat, in patients with ascites, and in women compared with men. With CT, the diameter of a normal appendix ranges from 3 to 10 mm. In one study, the normal appendix had a diameter more than 6 mm in 42% of cases. Therefore, an appendiceal diameter more than 6 mm is not alone sufficient for the diagnosis of acute appendicitis.
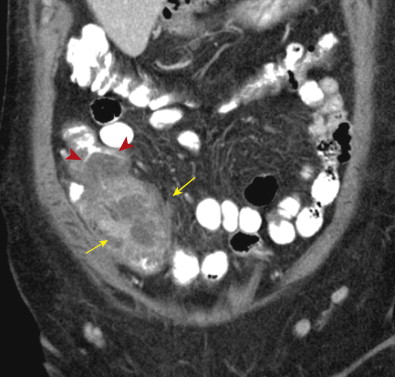
CT findings of appendicitis may include appendiceal distention, wall thickening, appendicolith, periappendiceal fat stranding, periappendiceal fluid, and abscess ( Figs. 56-14 to 56-16 ). In early or mild cases, the appendix commonly appears as a fluid-filled, minimally distended tubular structure measuring 5 to 6 mm in diameter. At this stage, the periappendiceal fat may have a normal appearance ( Fig. 56-17 ).
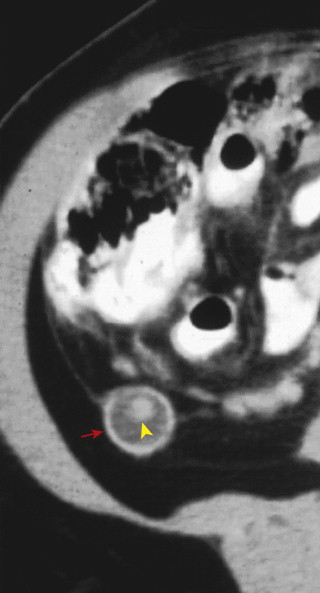
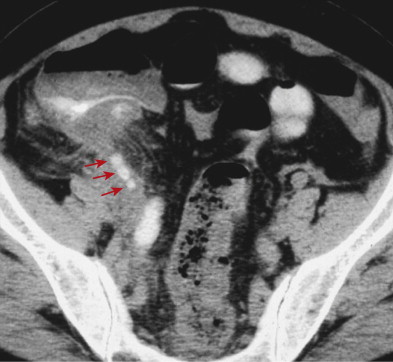
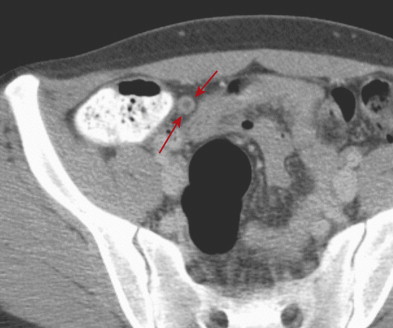
Over time, the appendix continues to distend, often measuring 7 to 15 mm in diameter. The appendiceal wall thickens circumferentially and enhances after intravenous (IV) contrast administration. The mural enhancement may be homogeneous or may exhibit a so-called target sign ( Fig. 56-18 ). Periappendiceal inflammation, seen as stranding in the adjacent fat, is present in most patients. Lack of IV contrast material limits visualization of mural thickening and mural enhancement in mild cases of appendicitis. As a result, noncontrast CT may result in a higher rate of false-negative interpretations. Many investigators consider periappendiceal inflammation a necessary imaging finding when diagnosing acute appendicitis with unenhanced CT imaging.
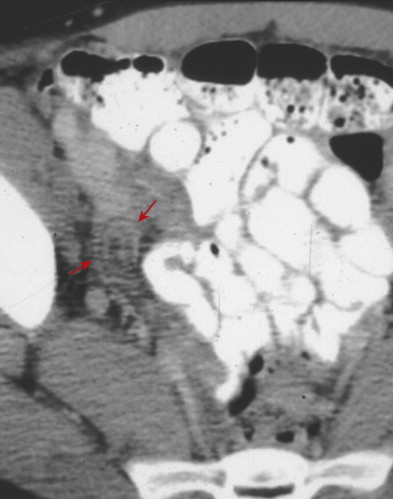
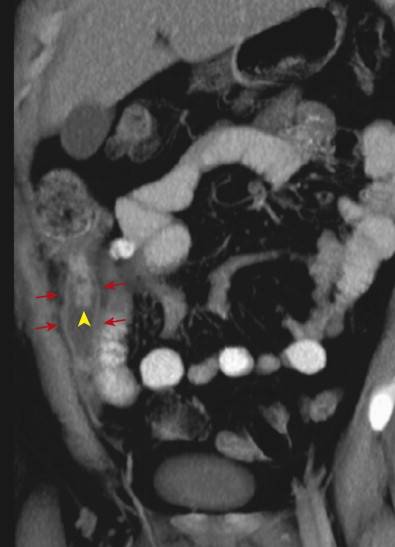
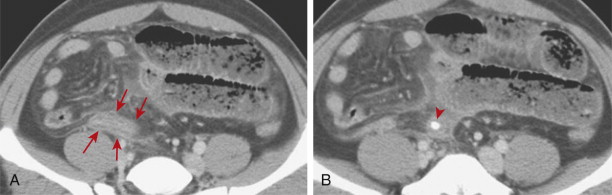
Inflammation of the appendix may also cause secondary reactive thickening of adjacent structures, including the wall of the terminal ileum or cecal caput. The cecal arrowhead sign refers to a triangular or arrowhead configuration of oral contrast material funneling into a focally thickened cecum, pointing toward the appendiceal orifice ( Fig. 56-19 ). The cecal bar sign refers to linear inflammatory soft tissue at the base of the appendix that separates the contrast-filled cecum from the appendix. With perforated appendicitis, extraluminal air, marked ileocecal wall thickening, localized lymphadenopathy, pericecal phlegmon or abscess, peritonitis, and/or small bowel obstruction may be present ( Fig. 56-20 ).
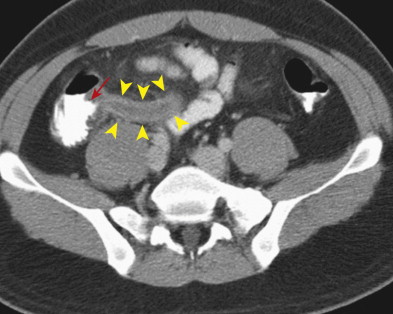
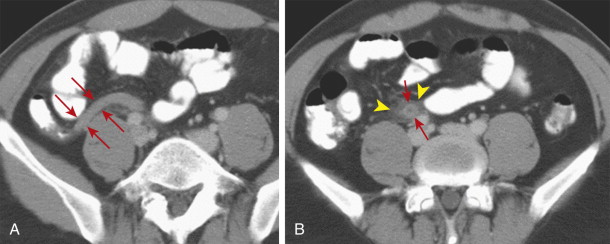
Appendicitis may also be confined to the distal appendix, a condition referred to as distal or tip appendicitis ( Fig. 56-21 ). Distal appendicitis (in which at least 3 cm of the proximal appendix is normal) may affect as many as 8% of patients with appendicitis who undergo CT.
Computed Tomography Protocols.
Conventional CT protocols for evaluating patients with suspected appendicitis incorporate abdominal and pelvic imaging after administration of oral and IV contrast material. Many studies have focused on alternate CT protocols in an attempt to reduce radiation dose to the patient, eliminate the patient risks associated with contrast administration, and enable earlier scanning rather than waiting for bowel transit of oral contrast material. Investigated strategies include scanning without IV or oral contrast, using rectal contrast, targeted imaging of the right lower quadrant, and reduced radiation dose scanning techniques.
CT protocols may not include oral or IV contrast. A major advantage of this approach is the ability to scan symptomatic patients immediately without having to wait for oral contrast material ingestion and transit to the cecum. Eliminating IV contrast means avoiding risks of adverse reaction and nephrotoxicity. Noncontrast scans also have lower costs.
Without oral contrast, disadvantages include potential misinterpretation of another structure (e.g., a loop of bowel) as the appendix, or misinterpretation of an abnormal appendix or abscess as a bowel loop. Relative to a contrast-filled appendix, finding the normal appendix without oral contrast is more difficult. Finally, identification of bowel thickening and luminal narrowing, two hallmarks of GI disease on CT, is often very limited without contrast material filling the bowel lumen.
Without IV contrast, disadvantages include limited assessment of wall thickening, inability to assess enhancement, limited differentiation of the appendix from blood vessels, reduced sensitivity for perforation or complication, and a lesser ability to make an alternate diagnosis. Identification of wall thickening and enhancement is often critical in patients in whom fat stranding is not detected, either because there is a paucity of retroperitoneal fat or because appendicitis is early or mild. One study found that 15% to 22% of patients with proven appendicitis had no detectable periappendiceal inflammation on CT.
Administration of rectal contrast material permits rapid filling of the cecum, with appendix identification in a large majority of cases. In a study by Rao and associates, use of a focused right lower quadrant technique with rectal contrast material enabled detection of the normal appendix in 94% of patients without appendicitis and visualization of part or all of the abnormal appendix in 96% of patients with acute appendicitis. In their study, 73% of the normal appendices filled with air or rectal contrast. Disadvantages of the rectal contrast technique include the potential for increased patient discomfort, inability to instill rectal contrast material in patients with contraindications to its use, and risk of causing a hydraulic pressure effect and rupturing the appendix. This technique may also fail if there is leakage of contrast material onto the CT table or if there is inadequate opacification of the cecum. Wise and co-workers reported that colonic contrast material was not successfully advanced into the cecum in 18% of 100 patients who underwent focused appendiceal CT, usually because of abundant stool in the right colon. One study reported that rectal contrast use resulted in a reduced length of patient stay in the emergency room without significant impact on patient satisfaction or comfort.
The goal of focused scanning is to reduce the portion of the abdomen and pelvis that is scanned, thereby reducing overall radiation dose to the patient. Although early studies showed that focused techniques may have comparable diagnostic accuracy, later investigations found an overall reduction in diagnostic performance. By using a focused technique, the appendix may not be visualized or may only be partially visualized. In addition, evidence of an alternate diagnosis may be excluded from the field of view. Brassart and colleagues compared scanning of the whole abdomen and pelvis with focused scanning below the iliac crests in the same set of 152 adult patients by generating two sets of images from the raw data. They found that the focused technique resulted in exclusion of the appendix from view in 5% of patients and a reduced likelihood of giving a correct diagnosis (from 78%- 68%). Common alternative diagnoses include acute gynecologic disorders, inflammatory bowel disease, small bowel obstruction, infectious disorders of the GI tract, and urinary tract conditions.
As of 2013, the consensus position of the American College of Radiology (ACR) expert panel is that IV contrast is preferred but not mandatory when using CT to evaluate for suspected appendicitis in adult patients. The use of oral or rectal contrast is left to institutional preference. For atypical presentations in patients older than 14 years, the use of IV contrast is relatively more valuable. In this group, ultrasound evaluation may be of similar value to noncontrast CT. In pregnant patients and children younger than 14 years, ultrasound is considered first-line imaging. When CT is used, IV contrast is preferred, whereas oral and rectal agents are again left to institutional preference. Magnetic resonance imaging (MRI) evaluation is gaining popularity as the technology evolves, particularly in pregnant patients or those in whom US is inconclusive.
Ultrasonography.
Puylaert’s introduction of the graded compression ultrasound technique in the 1980s has substantially improved sonographic identification of the appendix. US is widely available, noninvasive, relatively inexpensive, and poses no ionizing radiation risk to the patient. This latter feature is a major consideration in the most radiation-sensitive individuals, children and pregnant women.
Important limitations of US are reduced sensitivity in obese patients, inability to image beyond bowel gas, technical difficulties in patients with severe pain, and dependence on operator skill. Poor penetration in the obese population limits widespread use of this technique in North America and parts of Europe. Although some authors have reported that the normal appendix is visible in 64% to 72% of patients, others have noted a visualization rate of only 0% to 4% in adult patients, regardless of technique.
The reported diagnostic accuracy of ultrasound varies, depending on the patient population studied. A meta-analysis comparing CT and US in a broad age range found an overall ultrasound sensitivity of 78% and specificity of 83%. A large study of children aged 3 to 18 years found sensitivity and specificity of 73% and 97%, respectively.
Adherence to optimal scanning techniques is critical. Sagittal, transverse, and oblique imaging should be performed in the abdomen and pelvis, with the addition of a transvaginal examination in women in whom the diagnosis is not clear following the transabdominal approach. A high-frequency linear or curvilinear transducer may be used, depending on the patient’s body habitus. A high-frequency transducer typically offers the best image resolution, but a curvilinear probe may be better suited for imaging patients with a poorly compressible right lower quadrant bowel, large patients with their cecum and appendix located deep within the pelvis, patients with poorly defined right lower quadrant anatomy, and patients with a retrocecal or perforated appendix.
The technique of graded compression should be used to displace or compress gas-filled bowel loops in the field of view. This maneuver aids identification of the maximal point of tenderness while helping differentiate abnormal, noncompressible bowel loops or the inflamed appendix from those normally compressible structures. Also, the maintenance of slow and gentle pressure facilitates completion of the examination in uncomfortable and apprehensive patients.
Color Doppler evaluation may add valuable information by demonstrating hyperemia in the inflamed appendix or bowel wall. This can aid diagnosis in patients with equivocal gray-scale results.
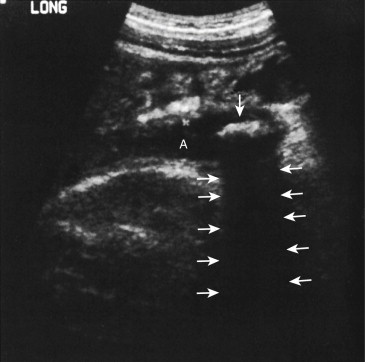
Sonographically, the abnormal appendix appears as a blind-ending, noncompressible, tubular structure larger than 6 mm in diameter with a laminated wall ( Figs. 56-22 to 56-24 ). Progressive ischemia and infarction of the appendiceal wall lead to focal or diffuse loss of definition of the wall layers. Hyperemia may be demonstrated on color Doppler evaluation, but decreased or absent flow may be seen in cases of gangrenous appendicitis. With perforation, localized disruption of the appendiceal wall may be seen, and extraluminal pockets of gas may be present. Appendicoliths appear as rounded echogenic foci with clean distal acoustic shadowing, and their presence is highly associated with appendicitis.
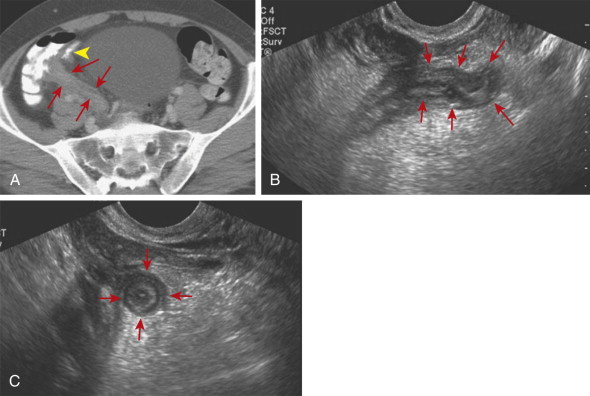
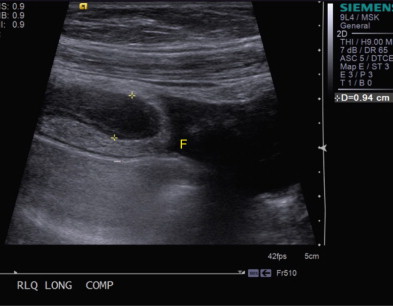
In addition to the appendiceal findings present in acute appendicitis, recognition of characteristic periappendiceal findings can aid in its diagnosis and differentiation from other conditions. Inflammation of the periappendiceal fat appears as an echogenic region that may cause mass effect and separate the inflamed bowel segment(s) from surrounding structures. Periappendiceal phlegmon appears as hypoechoic, poorly marginated regions within the fat adjacent to the appendix, and abscesses can be recognized as focal collections of fluid that may or may not contain gas. Reactive inflammatory thickening of the cecal or terminal ileal wall may also be present in patients with appendicitis.
Despite performance of optimized US examinations, US sensitivity for diagnosing perforated appendicitis is lower than that for nonperforated appendicitis. Overall, the noncompressible appendix is visible in only 38% to 55% of patients with perforated appendicitis.
As of 2013, ACR Appropriateness Criteria guidelines recommend ultrasound as the first-choice imaging technique in pregnant women and children younger than 14 years with suspected appendicitis.
Magnetic Resonance Imaging.
Recent advances in MRI technology have led to an increased role in the setting of suspected appendicitis. Superior soft tissue contrast resolution, multiplanar imaging capability, and ability to scan without contrast administration make MRI an attractive choice. Unlike CT, MRI does not involve the use of ionizing radiation. Drawbacks to the use of MRI include relatively long imaging times, higher cost, higher dependence on technique for good results, and reduced availability relative to CT.
Successful MRI protocols limit motion artifact and emphasize soft tissue contrast in the region of the appendix. Published protocols typically include single-shot fast spin-echo (SSFSE) sequences with T2 weighting in axial, coronal, and sagittal planes, at least one plane with SSFSE T2 with fat suppression, and one or more planes of T1-weighted imaging. Multiple studies have reported the highest appendix visualization rate with T2-weighted images. Others have shown improved sensitivity for acute appendicitis by including a diffusion-weighted sequence.
With MRI, the normal appendiceal walls are isointense to muscle on T1- and T2-weighted images. Luminal contents such as air are typically T1 and T2 hypointense. In pregnant patients, the appendix tends to migrate superiorly in the abdomen over time, often extending superior to the iliac crest by the third trimester.
MRI findings of acute appendicitis include a dilated appendix (>7 mm) filled with T2 hyperintense fluid, wall thickness more than 2 mm, and T2 hyperintense inflammation or fluid in the periappendiceal fat ( Fig. 56-25 ). The thickened appendiceal wall may also be hyperintense on T2-weighted imaging. MRI is indeterminate if the appendix measures 6 to 7 mm in diameter and contains T2 hyperintense fluid without evidence of wall thickening or a periappendiceal fluid signal.
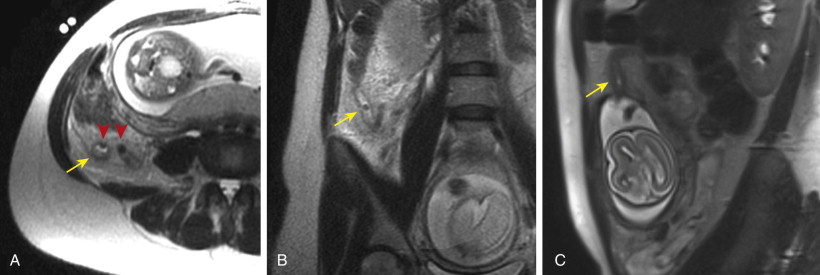
Although reduction or elimination of ionizing radiation is always desirable, the use of MRI for suspected appendicitis has grown most rapidly in patients for whom radiation concerns are most pronounced, notably pregnant women and pediatric patients. US remains the initial imaging test of choice in these patients, but the use of MRI has been growing in popularity, particularly when ultrasound examinations are inconclusive. At one institution, integrating MRI into the workup for suspected appendicitis in pregnant women resulted in a 47% reduction in the negative laparotomy rate (from 55%-29%). Some early studies comparing the two modalities have found better overall diagnostic performance with MRI, suggesting that MRI use may continue trending upward.
The clinical diagnosis of acute appendicitis can be more challenging in pregnant patients because of anatomic considerations, presence of leukocytosis in otherwise normal patients, and wide range of alternate pathologies. Early studies reported right upper quadrant pain to be the typical presentation of acute appendicitis during pregnancy, with the appendix migrating superiorly as the uterus enlarges. However, more recent investigations have found that right lower quadrant pain remains the most common presenting symptom, even in this patient population.
Despite early reports, most recent studies have found poor sensitivity for ultrasound in the diagnosis of acute appendicitis during pregnancy. In a large study by Pedrosa and colleagues, the normal appendix was only visualized in 2 of 126 pregnant patients without appendicitis (<2%). In patients with appendicitis, sensitivity was only 36% (5 of 14). In another smaller study, US could not identify the appendix in 92% of pregnant patients.
Theoretical risks to the fetus of MRI during pregnancy include heat deposition, acoustic damage, and gadolinium toxicity. When MRI heat deposition was studied in a pig model, results showed no significant change in amniotic fluid temperature. During an MRI examination, rapid current switching within the gradient coils creates substantial acoustic noise. In clinical use, maximum noise tends to be generated during echoplanar imaging (e.g., diffusion sequences) because of the high-gradient amplitudes and rapid switching required. Despite the theoretical concerns, studies following children exposed to echoplanar MRI in utero did not demonstrate a significant increase in postnatal disease or disability. MRI vendors have begun introducing scanners with reduced acoustic noise. This may help alleviate safety concerns while improving patient comfort and, as a result, motion degradation. Despite the theoretical risks of MRI to the fetus, no studies have shown deleterious long-term effects. MRI has long been used in the obstetric population to evaluate fetal anomalies, without known adverse effects.
Gadolinium toxicity has garnered attention as our understanding of nephrogenic systemic fibrosis continues to evolve. In the pregnant female, IV gadolinium-based contrast crosses the placenta and is filtered by the fetal kidneys. Subsequently, the gadolinium agent is excreted into the amniotic fluid, where some of it remains in contact with the fetus for a prolonged period. Because there is more time inside the body, there is more time for the chelated agent to break down and release the free gadolinium, which has been implicated in toxicity events.
Based on this physiology, the U.S. Food and Drug Administration has classified gadolinium-based contrast agents as category C agents. The 2013 ACR guidance document on safe MR practices stated that “The risk to the fetus of gadolinium-based MR contrast agent administration remains unknown and may be harmful.”
IV gadolinium is not routinely used when using MRI to evaluate for suspected appendicitis. Fortunately, recent studies have demonstrated a similar level of diagnostic accuracy using contrast-enhanced CT and noncontrast MRI to detect acute appendicitis in the pregnant population. A meta-analysis by Blumenfeld and associates noted sensitivity, specificity, and positive and negative predictive values of MRI for diagnosing appendicitis during pregnancy as 95.0%, 99.9%, 90.4%, and 99.5%, respectively, when scans of diagnostic quality were reviewed.
Differential Diagnosis
The presence of inflammatory change adjacent to the cecum is typical of acute appendicitis but is not specific for the diagnosis. The differential diagnosis includes cecal or ileal diverticulitis, Crohn’s disease, mesenteric adenitis, epiploic appendagitis, inflamed Meckel’s diverticulum, infectious or ischemic ileitis, typhlitis, perforated cecal carcinoma, and pelvic inflammatory disease.
Identification of the normal appendix can facilitate an appropriate alternate diagnosis. The distinction can be critical because, unlike appendicitis, many of the other conditions are treated conservatively.
Mesenteric Adenitis
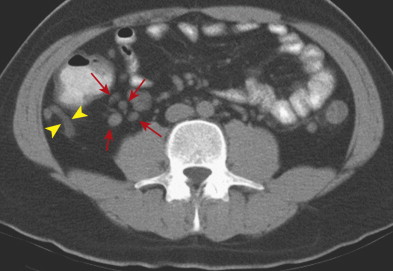
Mesenteric adenitis is the second most common cause of right lower quadrant pain after acute appendicitis, accounting for 2% to 14% of discharge diagnoses in patients suspected of having appendicitis.
Primary mesenteric adenitis is diagnosed by CT or US when there are more than three right-sided mesenteric lymph nodes 5 mm or larger without an identifiable acute inflammatory process or with mild (<5-mm) terminal ileal wall thickening. Mesenteric nodes should not be considered responsible for the patient’s symptoms unless a primary intestinal inflammatory lesion is excluded. Underlying infectious terminal ileitis is thought to be responsible for most cases of primary mesenteric adenitis.
In secondary mesenteric adenitis, the enlarged mesenteric lymph nodes are related to an identifiable underlying condition. Possible causes include acute appendicitis, infectious enteritis or enterocolitis, diverticulitis, Crohn’s disease ( Fig. 56-26 ), celiac disease, and neoplasm.