16
Diseases of the Thoracic Aorta
Medical and surgical treatment as well as recently developed interventional catheter procedures for the treatment of aortic diseases requires precise delineation of the pathological disorders. Concurrent with the development of these new techniques, the emergence of new imaging modalities such as magnetic resonance imaging (MRI), spiral computed tomography (CT), and transesophageal echocardiography (TEE) have revolutionized our ability to diagnose these complex conditions. Once considered the gold standard, angiography remains important only when other techniques are inconclusive.
Acquired diseases of the aorta such as ectasia, aneurysm, dissection, and its variants occur as a result of degenerative processes in older persons. These processes are accelerated in persons who have underlying connective tissue disorders such as Marfan syndrome and in those with hypertension and atherosclerosis. Of these entities, acute aortic dissection or rupture may represent a surgical emergency. Aortic trauma also results in an acute emergency requiring the most expeditious diagnosis and surgical management. Aortic arteritis syndromes are caused by inflammatory diseases such as giant cell arteritis and Takayasu arteritis, which may lead to aortic insufficiency or obstructive lesions. Aortitis also occurs during late stages of syphilis. Complications of catheter and surgical procedures also may require surgical intervention. Recognition of these complications has become more important as more procedures are performed. Secondary involvement of the aorta by neoplasms, abscesses, and hematomas are a challenge to the clinician. Modern imaging techniques have become essential in the diagnosis and management of these entities.
Congenital diseases of the aorta constitute a smaller segment of the population with aortic disease.1,2 The most common entities are preoperative and postoperative coarctation of the aorta and patent ductus arteriosus. Other uncommon disorders occasionally are encountered, including vascular rings, cervical aortic arch, interruption of the aortic arch, and anomalous origin of a pulmonary artery. Pulmonary artery sling and ductus arteriosus sling are rarely encountered. Bronchial arteries are dilated in cyanotic congenital heart disease with pulmonary atresia and may be hemodynamically significant as a source of pulmonary blood flow. Coil embolization may be necessary before surgical correction of these entities. Pulmonary sequestrations are supplied by vessels arising from the aorta and may be treated by coil embolization.
 Aortic Dissection
Aortic Dissection
Aortic dissection consists of an intimal tear in the wall of the aorta that causes separation of the intramural layers, usually between the middle and outer thirds of the media, creating a false lumen (Fig. 16-1). Blood in the false lumen may reenter the true lumen via a second intimal tear, or it may rupture through the adventitia into the periaortic tissues. Most commonly, dissections are believed to be caused by cystic medial necrosis. Proximal dissections tend to occur in younger persons who often are found to have Marfan syndrome (Figs. 16-2 and 16-3). Less commonly, inherited connective tissue disorders such as osteogenesis imperfecta, Turner syndrome, and Ehlers-Danlos syndrome may be associated with proximal dissections. Distal dissections occur more commonly in older patients with hypertension. Other less common causes include atherosclerosis, trauma, syphilis, idiopathic kyphoscoliosis, and congenital heart defects, such as coarctation and pseudocoarctation of the aorta. Approximately 2,000 new cases of aortic dissection are diagnosed each year in the United States.34 The male-to-female ratio is approximately 2:1, with a peak incidence in the sixth and seventh decades of life.
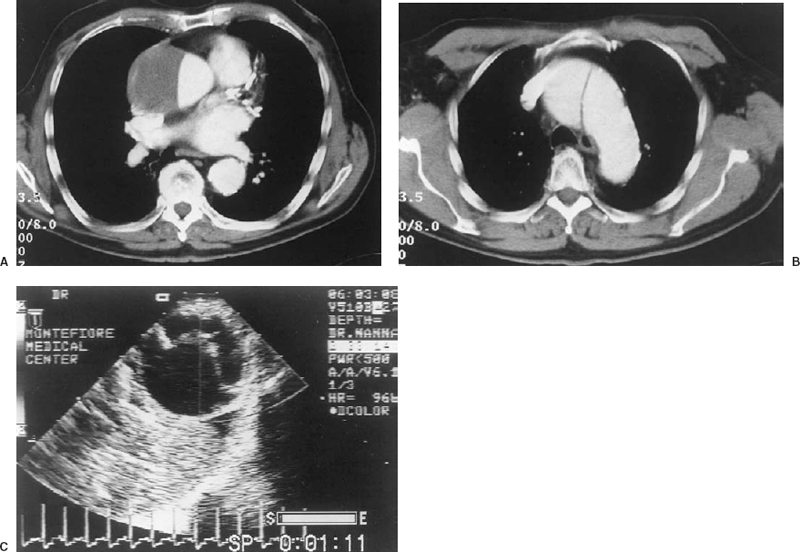
FIGURE 16-1. Type I dissection of the aorta in a 74-year-old man. The dynamic spiral computed tomography (CT) shows the characteristic flap in a type I dissection involving the ascending aorta (A) and the proximal aortic arch (B). The brachiocephalic vessels were not involved. Calcification of the wall of the aorta is noted. Transesophageal echocardiogram in a different patient with a type I dissection showing the flap in the descending aorta (C). On the real-time image, the flap oscillates in the lumen of aorta. The true lumen is closest to the transducer and the false lumen is farther away. The echogenicity beyond the aorta represents extravasated blood in the mediastinum.
Two major classifications of aortic dissection are widely used. The older classification, introduced by DeBakey et al., divides aortic dissections into type I, which includes those involving the ascending and descending aorta (Fig. 16-1); type II, which involves the ascending aorta only; and type III, which involves the descending aorta (Fig. 16-4).5 A mnemonic for this classification is “BAD”: type I = both aortas, type II = ascending aorta and type III = descending aorta. Another classification, introduced by Dailey et al., and often called the Stanford classification, divides aortic dissection into two groups. Dailey’s type A includes proximal dissections as well as those distal dissections that extend retrograde to involve the arch and ascending aorta. Type B includes dissections of the distal aorta without proximal extension.6
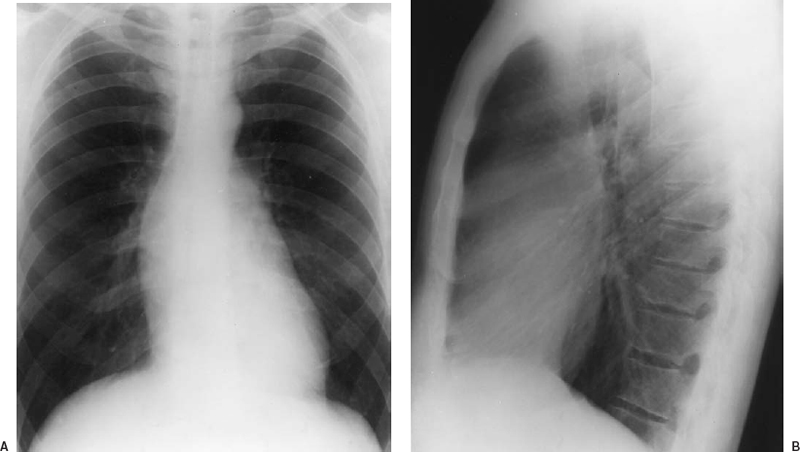
FIGURE 16-2. Findings on chest roentgenography in a man with Marfan syndrome. A: Frontal view. B: Lateral view. Marked dilatation of the root of the aorta produces a double density within the cardiac silhouette (pseudo left atrial enlargement). The normal angle formed between the ascending aorta and the base of the heart is effaced by the dilated ascending aorta. The aorta also displaces the main pulmonary artery superiorly and laterally, producing a bulge in the pulmonary artery segment simulating pulmonary artery dilatation. In the lateral film, the dilated aortic root obliterates the retrosternal clear space. Thus, the lateral film shows that the double density noted on the frontal view is due to the dilatation of the aortic root rather than enlargement of the left atrium. Note that the dilatation of the root of the aorta does not extend into the aortic arch. Mild left ventricular enlargement is present (same patient as in Fig. 16-3). (Courtesy of Spindola-Franco H, Fish BG, eds. Radiology of the heart: cardiac imaging in infants, children, and adults. New York: Springer-Verlag, 1985:203.)
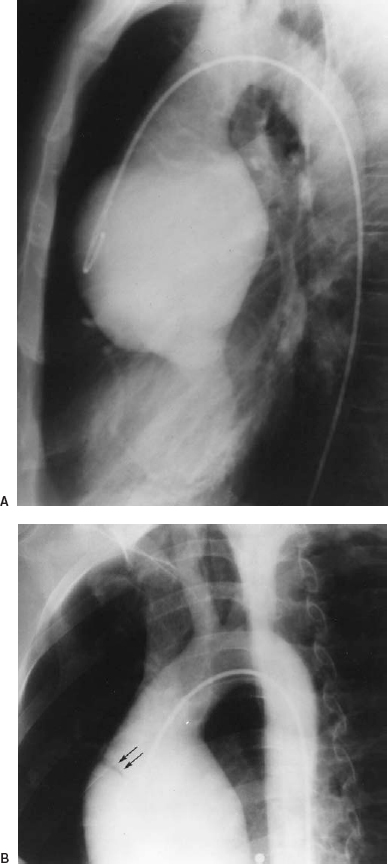
FIGURE 16-3. Aortic insufficiency in an asymptomatic patient with Marfan syndrome. The aortogram. A: Lateral view. B: Shallow left anterior oblique (LAO) view shows that the root of the aorta is dilated symmetrically and bulbous, with effacement of the semilunar sinuses. Aortic insufficiency is present. In (B) a tear is demonstrated in the anterolateral wall (arrows). The tear was not detected on the lateral film, which emphasizes the requirement to look carefully for tears, using multiple views as necessary. This example is a “silent aortic dissection” (same patient as in Fig. 16-2). (Courtesy of Spindola-Franco H, Fish BG, eds. Radiology of the heart: cardiac imaging in infants, children, and adults. New York: Springer-Verlag, 1985:204.)
Important variants of aortic dissection are caused by penetrating ulcers and by intramural hemorrhage (IMH) (Fig. 16-5).7 Penetrating ulcers result from erosion of atherosclerotic plaque with eventual violation of the internal elastic lamina.8 Penetrating ulcers occur most often in persons who have extensive atherosclerotic disease, but not in persons with Marfan syndrome or other diseases of connective tissue. Ulcers affect the mid-portion to distal portion of the descending thoracic aorta almost exclusively and do not result in valvular, pericardial, or neurovascular complications that occur with aortic dissection. The ulcer has a nipple-like configuration extending beyond the intima and is surrounded by hematoma. The process may be contained spontaneously or may progress to false aneurysm formation or aortic rupture (Fig. 16-6).
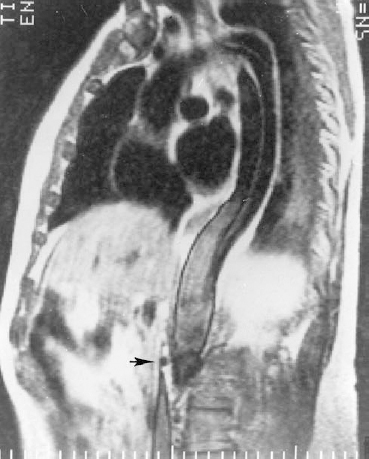
FIGURE 16-4. Complications of DeBakey type III aortic dissection in a patient who presented with acute abdominal pain. The left anterior oblique (LAO) plane of a T1-weighted spinecho image shows two flaps distal to the left subclavian artery. One false lumen is confined to the distal aortic arch and proximal descending aorta. The other extends into the abdomen, compromising the superior mesenteric artery (arrow). Ischemia of the gut caused acute abdominal pain in this patient. Angiography was not successful because the catheter could not be advanced into the lumen of the aorta.
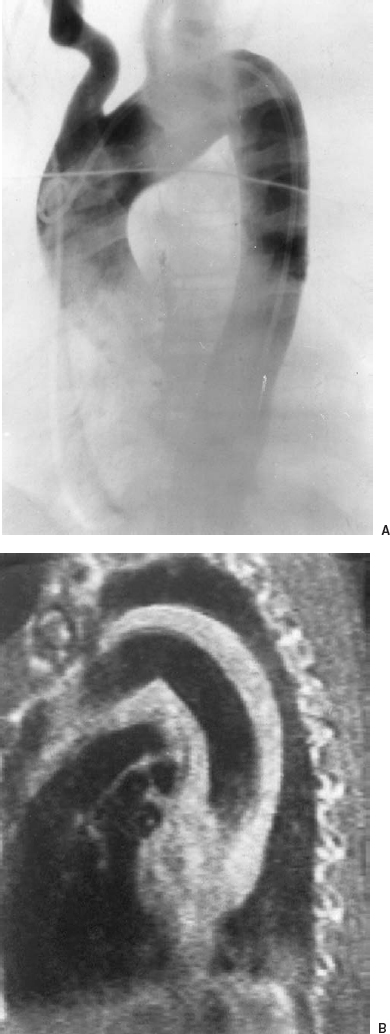
FIGURE 16-5. Angiogram and magnetic resonance imaging (MRI) in a patient with intramural hemorrhage (IMH). A: Subtraction film from the left anterior oblique (LAO) view of an aortogram. B: T1-weighted spin-echo pulse sequence MRI in the sagittal plane. In (A) the aortic lumen is normal with no intimal flap or leakage, whereas in (B) the high signal intensity and increased thickness of the aortic wall indicate intramural hemorrhage. The increased signal intensity indicates a subacute process. Acute hemorrhage would be isointense and may not be separated from the wall of the aorta. Blood is also present in the mediastinum, considered a typical feature of IMH.
IMH may result from rupture of aortic vasa vasorum.9 IMH differs from classic dissection in that hematoma forms within the aortic wall, but an intimal tear is not present.10,11 Intramural hemorrhage may be the first stage in the development of aortic dissection, the second stage being an intimal tear that allows blood to flow into the false lumen, extending the plane of dissection.12 Complications of IMH are the same as with aortic dissection, but they are significantly less common with IMH than with aortic dissection.
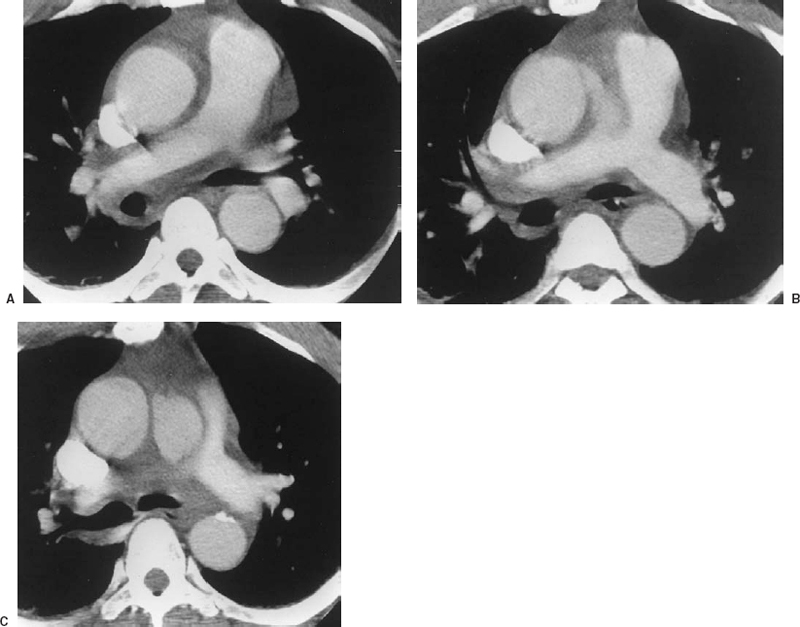
FIGURE 16-6. Acute perforation of an atherosclerotic ulcer in a 54-year-old man with sudden onset of severe chest pain. Frames from a dynamic spiral CT scan. A: The lowest slice, the ascending aorta is dilated, but no dissection is noted. B: 1 cm higher, a jet of contrast exits the aortic lumen into the mediastinum. C: 1 cm above (B), a collection of contrast is noted just under the aortic arch. The surrounding fat is dense (“dirty fat sign”), indicating infiltration with blood. These findings are consistent with rupture of an atherosclerotic plaque. Soon after the scan, the patient became more unstable and was taken to the operating room. At surgery, an aortic dissection and a hemopericardium were found, indicating that propagation of the pathologic process had occurred, accounting for the sudden deterioration after the scan.
Clinical features
Aortic dissection often presents with severe chest pain (anterior or posterior) with a tearing character. Pain is maximal at onset and changes in location as the dissection propagates. Painless (“silent”) dissection is rare (Fig. 16-3). Acute complications include aortic insufficiency and congestive heart failure. Aortic insufficiency occurs in up to 50% of patients who undergo type A dissection secondary to either widening or distortion of the aortic annulus, causing failure of coaptation of the valve leaflets, or torn leaflets. Congestive heart failure may result from sudden onset of severe aortic insufficiency. Syncope as a presenting symptom may be associated with rupture into the pericardial cavity and cardiac tamponade. Physical findings with aortic dissection include shock with normal to elevated blood pressure and pseudohypotension or pulse deficits secondary to dissection of the brachiocephalic vessels. Myocardial infarction occurs in a small percentage of patients with proximal dissection, secondary to involvement of the coronary arteries. Neurological findings and renal and mesenteric infarctions occur as a result of occlusion of related vessels. Left ventricular hypertrophy is commonly noted on electrocardiography. Aortic dissection should be suspected in patients with chest pain and no electrocardiographic signs of myocardial ischemia.
Imaging modalities
On chest roentgenography, the characteristic findings of aortic dissection are a widened aortic contour and a “calcium sign” consisting of a greater than 5 mm separation of the calcification of the aortic arch from its outer contour. A left pleural effusion may be present, indicating leakage into the pleural space. The trachea may be deviated. Importantly, the chest roentgenogram may be normal in aortic dissection.
TEE (Fig. 16-1) and MRI are both very sensitive in the diagnosis of aortic dissection.13,14 Advantages of TEE include the availability of this modality at the bedside, enabling diagnosis in acutely unstable patients. TEE is useful in proximal and distal dissections. A disadvantage is its inability to delineate the branches of the aortic arch accurately. In addition, TEE is an invasive procedure.
If available, MRI is the procedure of choice in persons with aortic dissection who are relatively stable and can tolerate a longer procedure (Fig. 16-4). The advantages of MRI include its ability to determine the exact extent and site of origin of the dissection and its anatomic relationships with neighboring organs. MRI also allows the diagnosis of associated aortic insufficiency and leakage. By using MRI, thrombus or flow in the false lumen can be detected. Cine MRI allows visualization of the oscillations of the flap separating the false lumen from the true lumen, which is a hallmark of aortic dissection.
Spiral and ultrafast CT are extremely useful in the acute assessment of aortic dissection when MRI is not available or when the patient cannot tolerate the time required by the MRI examination (Fig. 16-1). Ultrafast CT is available in only a few centers, whereas spiral CT has replaced standard CT in many centers and often is staffed through the night. The shorter time taken to perform a CT study compared with the time taken by MRI should be weighed against the radiation exposure involved and the requirement to administer angiographic contrast medium. One limitation of CT relates to difficulty in timing the injection to enhance the false lumen.
MRI, spiral CT, TEE, and angiography are all complementary, and each has an ability to identify aortic dissection; however, angiography cannot detect IMH (Fig. 16-5).15 Atherosclerotic ulcers are detected by angiography, but the hematoma surrounding a penetrating ulcer may be missed. For this reason, angiography is not the primary diagnostic modality for these entities but should be used when the others are not definitive.
Treatment
Without treatment, aortic dissection is a lethal disease. Death occurs as a result of progression of the dissection, which causes damage to vital structures. Ninety percent of untreated patients die within one year, with the highest mortality occurring during the first week after the onset.16 Initial treatment for all patients includes beta-blockers and aggressive control of hypertension to prevent progression. Proximal dissections are surgically repaired early or even emergently because of the risk of catastrophic consequences such as acute aortic insufficiency, cardiac tamponade, and neurologic complications with even minimal progression. On the other hand, distal dissections may be treated medically unless they show signs of rupture, impending rupture, or renal or mesenteric compromise. Chronic dissections, which present 2 weeks or longer after onset, also may be treated medically because they already have passed the most dangerous period, suggesting a nonlethal outcome. Surgery of proximal dissections commonly consists of placement of a composite graft containing a prosthetic valve. The native ascending aorta is wrapped around the graft. The coronary arteries are reimplanted using a button of native aorta to facilitate the anastomosis. Conduit grafts also may be used to replace the aortic arch and descending aorta.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree