Diseases of the Uterus
This chapter deals with the imaging of the most common gynecologic conditions. Congenital anomalies of the female genital tract are included in Chapter 2. Obstetrical imaging is beyond the range of this text.
▪ MYOMETRIAL DISEASES
Leiomyoma (Fibroid)
Uterine leiomyomas are extremely common, benign, smooth-muscle tumors of the myometrium. Their prevalence has been quoted as high as 20% to 30% of women seen in clinical practice and 50% in autopsy series. They are rare in premenarchal girls, enlarge with pregnancy or other condition with elevated estrogens (such as endometrial hyperplasia and carcinoma, granulosa-theca tumors, and anovulatory states), and involute after menopause. They are seen more commonly in African American women than in white women.
The tumors consist of masses of smooth-muscle cells. Despite the colloquial name of fibroid, they are not fibrous neoplasms. The tumors are often multiple and vary in size from microscopic nodules to huge masses; lesions weighing dozens of pounds have been encountered. The tissue may be viable or may degenerate, becoming replaced by hyaline material, infarcted red necrotic tissue or fluid; extremely rarely, they may become infected and filled with pus. The tumors are biologically benign; they do not spread locally or metastasize. A small fraction (0.1% to 0.5%) may undergo malignant degeneration and become leiomyosarcomas.
Most of the tumors are mural; that is, they begin and expand within the myometrial wall. They may also be submucous (submucosal) or appear on the serosal surface. They may be pedunculated and grow entirely within the endometrial cavity or exist at the ends of external stalks and thus become adnexal masses. They infrequently form in the cervix, although fibroids in the endometrial cavity may prolapse through the external cervical os. Rarely, the tumors are encountered in the broad ligament. Extrauterine tumors may parasitize vessels from adjacent structures, including the ovaries and gut.
Fibroids are usually asymptomatic, but they may produce a variety of symptoms, including abnormal bleeding, pelvic pain, dyspareunia, infertility, urinary frequency, and abdominal enlargement. The symptoms are usually chronic, but occasionally there may be a sudden infarction of a leiomyoma or a pedunculated tumor may undergo torsion, either of which may cause severe acute pain. The masses may reach extremely large sizes and may cause partial obstruction of one or both ureters by compressing them between the mass and the bony pelvis or iliac arteries.
Most uterine leiomyomas require no treatment, but bleeding, pain, infertility, and other symptoms may prompt hysterectomy. If a patient desires to retain her uterus, sometimes a myomectomy can be performed. If the fibroid is submucous, the surgery may be hysteroscopic. Angioembolization is becoming more frequently used, as is high-intensity focused ultrasound. Finally, there have been recent developments in medical therapy in which gonadotropin-releasing hormone analogs are used to diminish circulating estrogen.
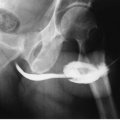
Plain radiographs may reveal leiomyomas as pelvic masses if they are sufficiently large. Amorphous or “popcorn” calcifications are seen in a minority of tumors. Hysterosalpingography and saline infusion hysterography reveal a variety of findings. Pedunculated serosal tumors, or small mural masses, may produce no abnormalities at all. Submucous lesions produce round-filling defects protruding from the uterine wall; occasionally, a pedunculated endometrial fibroid may look like a completely intraluminal polypoid filling defect. Mural tumors that reach several centimeters in size may distort the endometrial cavity in a variety of bizarre configurations. Large ones may enlarge the cavity (Fig. 19.1) by stretching it as it surrounds the expanding mass.
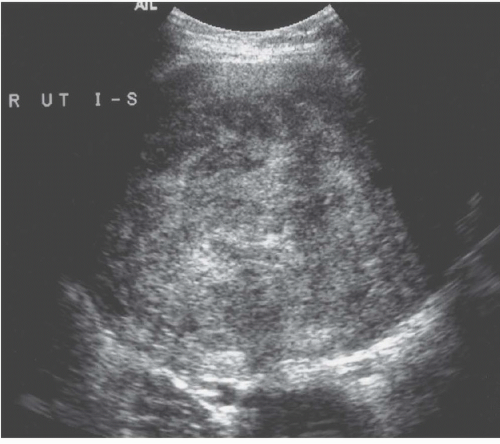
Ultrasound demonstrates most fibroids, except those that are small mural tumors or that are hard to detect because of an acutely flexed uterus. They are most often hypoechoic (Fig. 19.2), but they may be moderately echogenic or have a mixed pattern (Fig.19.3) and occasionally display an echogenic rim; shadowing may arise from the interface between regions of differing cellularity within the masses or at the border of the mass with adjacent normal tissue. Calcifications are seen as echogenic foci with associated shadowing. Rarely, the tumors have a whorled internal appearance. The endometrial space may be stretched, enlarged, and distorted by adjacent masses; and in the case of pedunculated endometrial fibroids, a relatively hypoechoic mass completely within the endometrial cavity may be seen. Pedunculated serosal fibroids may be difficult to distinguish from other adnexal masses; they are usually hypoechoic. Fibroids may appear cystic if they have undergone necrosis or degeneration. Viable lesions usually demonstrate considerable flow by Doppler studies. Features of adnexal masses suggesting that they may be fibroids include typical calcifications, a
close anatomic association with the uterus, and demonstration of a normal ipsilateral ovary.
close anatomic association with the uterus, and demonstration of a normal ipsilateral ovary.
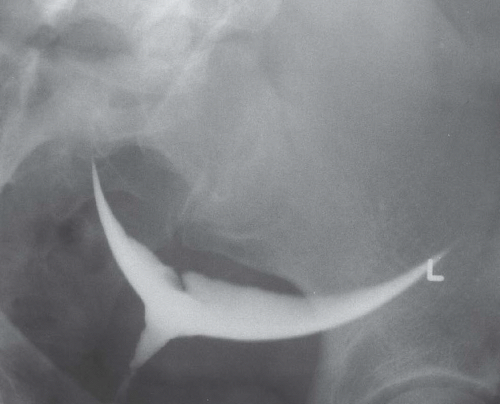 FIGURE 19.1. Large uterine fibroid. Hysterosalpingography reveals enlargement and a distortion of the endometrial cavity by a mural fibroid. |
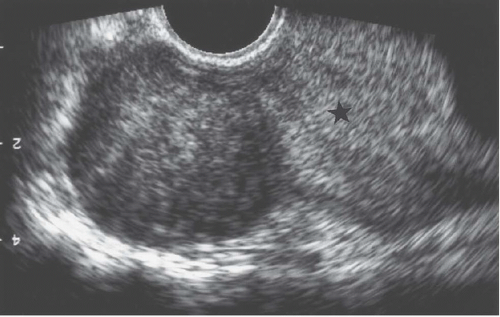 FIGURE 19.2. Exophytic fibroid. Transvaginal ultrasound reveals a mass that is less echogenic than the adjacent myometrium (star). |
During the first 6 months of pregnancy, serial ultrasound examinations may show enlargement of the fibroids. Acute degeneration, presumably from infarction, may be observed when serial examinations reveal some change in echo texture along with acute pain.
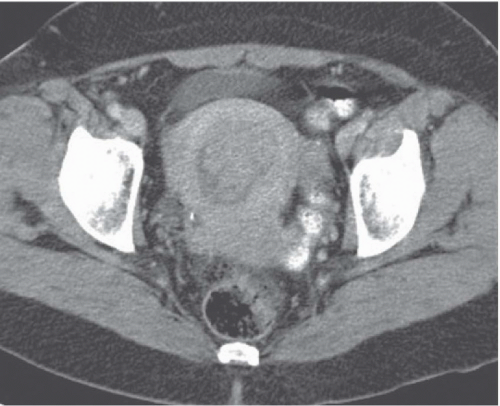
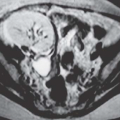
Computed tomography (CT) may delineate fibroids, but seldom as well as ultrasound and never as well as magnetic resonance imaging (MRI). Without intravenous contrast, fibroids present a variety of appearances. They may cause uterine enlargement, and there may be a lobulated appearance of the external uterine surface. Visualization of discrete masses is uncommon, but masses may be seen if there are pedunculated fibroids attached to the serosal surface. Rarely, the endometrial space may be distorted by a large tumor (Fig. 19.4). Usually, fibroids are isointense to normal myometrium; occasionally, they may be less dense (Fig. 19.5) if they have undergone necrosis or
degeneration. Rarely, they may demonstrate increased density if they have undergone recent hemorrhage, and they may display amorphous calcification with the characteristic popcorn appearance, or, less frequently, in a ringlike pattern. After angioinfarction, they may develop necrotic centers that occasionally contain small gas bubbles; these bubbles do not always connote infection.
degeneration. Rarely, they may demonstrate increased density if they have undergone recent hemorrhage, and they may display amorphous calcification with the characteristic popcorn appearance, or, less frequently, in a ringlike pattern. After angioinfarction, they may develop necrotic centers that occasionally contain small gas bubbles; these bubbles do not always connote infection.
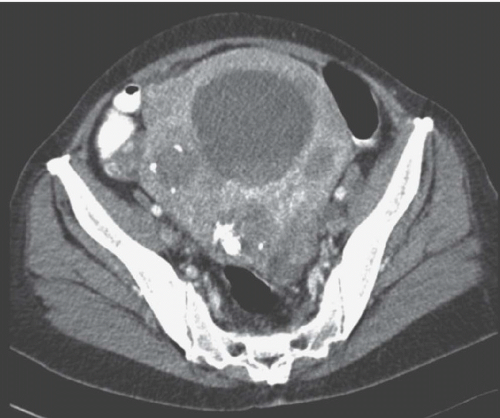 FIGURE 19.5. Uterine fibroid. Contrast-enhanced CT shows large calcified heterogeneously enhancing pelvic mass. Lucent regions are areas of necrosis. (Courtesy of Sherelle Laifer-Narin, M.D.) |
After the administration of intravenous contrast, fibroids may display a variety of appearances. The most characteristic is that of intense enhancement, but frequently they are isodense to normal myometrium and, if they are necrotic or degenerated, may not enhance at all. Very rarely, degenerated fibroids may appear as septated masses.
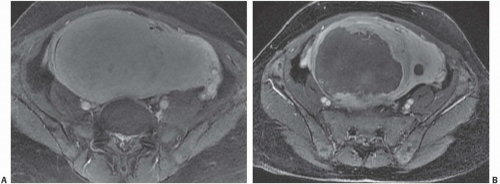
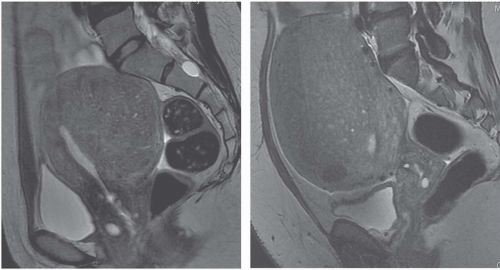
MRI is the most accurate of all imaging techniques for detecting and delineating the anatomy of fibroids. The tumors may display a variety of signal intensities; the most common of these is isointensity or hypointensity (compared with normal myometrium) on T1-weighted images and hypointensity on T2-weighted images (Fig. 19.6). Calcified regions have a very low intensity on all images. Extremely cellular tumors may appear slightly brighter on T2-weighted images, and cystic or degenerated regions appear bright. Lesions found to be very cellular by T2-weighted MRI are more likely to be successfully treated with gonadotropin-releasing hormone. The tumors occasionally display a bright rim on T2-weighted images, and their degree of enhancement on T1-weighted images following gadolinium administration is variable.
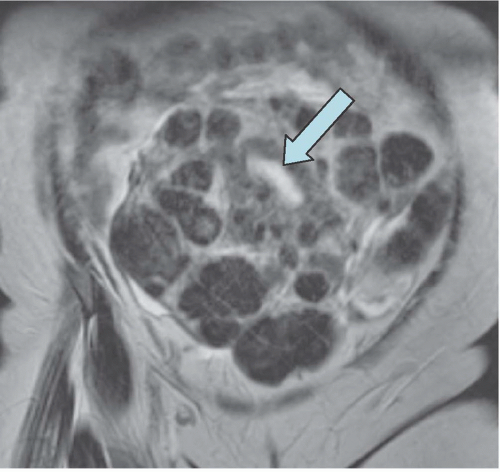 FIGURE 19.6. Uterine fibroids. Coronal T2-weighted MRI reveals large abdominopelvic mass. Low-intensity regions are areas of dense muscle; the bright region (arrow) is the endometrial cavity. |
MRI may be useful in distinguishing pedunculated fibroids from other adnexal masses. Demonstration of a normal ipsilateral ovary suggests that a mass may be of uterine origin. Low intensity on both T1- and T2-weighted images strongly suggests that the mass is a fibroid, although ovarian fibromas may also display this appearance. If a pedunculated fibroid is cystic or degenerated centrally, it may be more difficult to distinguish from other cystic or hemorrhagic adnexal masses.
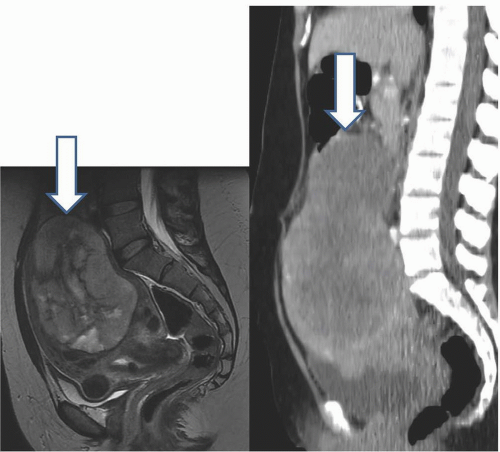
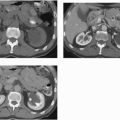
MRI is probably the most useful single imaging technique for evaluating fibroids for interventional treatment or myomectomy; it is the most accurate technique in identifying the size, number, location, and perfusion of the tumors (Fig. 19.7A). Before uterine arterial embolization or high-intensity focused ultrasound therapy, it provides particularly valuable information. Percent decrease in size and resolution of symptoms is inversely proportional to pretreatment tumor volume, and liquefied or necrotic tumors are less amenable to these therapies. Submucous location may be a relative contraindication, since passing a large sloughed tumor through the cervical canal may be painful and involve considerable bleeding, and a pedunculated adnexal fibroid may become detached from the uterus if it infarcts, and produce complications in the pelvis. Posttherapy analysis of infarcted tumor volume is helpful: the greater the fraction of the tumor that is not perfused, the more successful the treatment is likely to be (Fig. 19.7B).
Sarcoma
Uterine sarcomas are unusual, but aggressive tumors. They may be of a number of histologic types. Leiomyosarcomas are the most common; some authors feel that they arise from fibroids. There are other single-cell-type sarcomas that arise from any of the connective tumors in the uterus, including stromal tumors, fibrosarcomas, angiosarcomas, and others. Approximately as common as leiomyosarcomas are mixed mullerian tumors, which contain elements of both carcinoma (presumably from the endometrium) and sarcoma; the sarcomatous element may be of any of a variety of cell types.
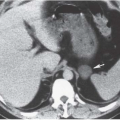
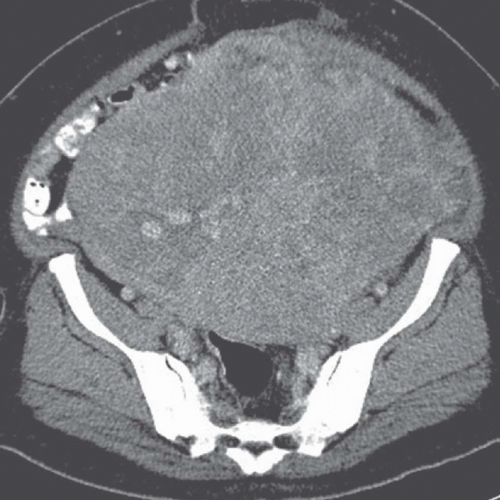 FIGURE 19.8. Uterine leiomyosarcoma. CT shows the tumor to be large and heterogeneous. It cannot be reliably distinguished from a large fibroid. |
Sarcomas often present with postmenopausal bleeding; there may be abdominal pain, enlargement of the uterus, and systemic symptoms such as weight loss. In patients known to have leiomyomas, rapid enlargement of the uterus or one of the tumors should suggest malignant transformation of a leiomyoma into a uterine sarcoma, especially if the patient is postmenopausal.
These tumors are often metastatic at the time of diagnosis and frequently recur after resection, even if systemic disease was not evident initially. Metastases appear in the pelvis, lung, upper abdomen, and other sites.
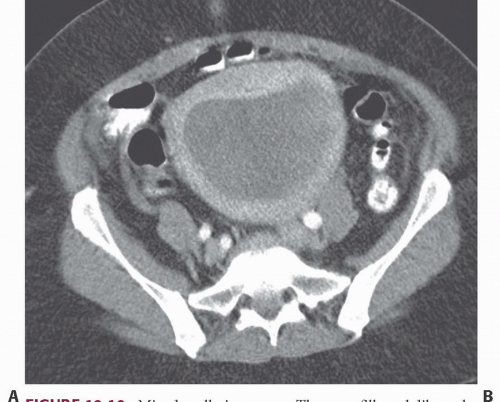 FIGURE 19.10. Mixed mullerian tumor. The mass fills and dilates the endometrial space, and cannot be distinguished from an endometrial carcinoma. |
Imaging studies do not always permit differentiation of uterine sarcomas from other tumors because they appear as masses on any imaging modality. Ultrasound may reveal them to be solid or cystic and septated. Doppler ultrasound may show low impedance and low resistive indices in sarcomas, but these features are seen in some leiomyomas as well. On CT, leiomyosarcomas are more likely to have necrotic regions than are leiomyomas, and they may be larger (Fig. 19.8) and have more bizarre calcification. Nevertheless, they are often difficult to distinguish from fibroids. Rapid increase in size suggests sarcomatous degeneration (Fig. 19.9), as does the demonstration of nodal or other metastases. Mixed mullerian tumors, or carcinosarcomas, often expand primarily within the endometrial space, and may be difficult to distinguish from large endometrial carcinomas (Fig. 19.10). MRI reveals leiomyosarcomas to be heterogeneous, especially on T2-weighted images. Those that arise in or near the endometrial lining may destroy the endometrial and subendometrial layers normally seen on T2-weighted MR images. The tumors are usually large and tend to have more irregular contours and indistinct borders than do leiomyomas. At the time of diagnosis, enlarged lymph nodes or metastases to the solid organs of the upper abdomen or lung may be seen.
▪ ENDOMETRIAL DISEASES
Adenomyosis
Adenomyosis, sometimes called endometriosis interna, consists of proliferation of the endometrium within the myometrium. It is believed to result from invagination of normal endometrial tissue into the myometrium or possibly from the development of mullerian rests. It may be focal, or it may appear diffusely throughout the uterus; areas of adenomyosis are always continuous with the endometrium and junctional zone. There is muscular proliferation within the myometrium in response to adenomyosis; however, the development of macroscopic cysts is rare.
Adenomyosis is an estrogen-sensitive condition. It is usually found in women with several children. In mild and asymptomatic forms, it is estimated to occur in 10% to 40% of women. It may produce dysmenorrhea, infertility, and menorrhagia, and may
coexist with endometriosis. It may be treated with danazol or other drugs or may require a hysterectomy; techniques for local ablation, including high-intensity focused ultrasound and arterial embolization, continue to evolve.
coexist with endometriosis. It may be treated with danazol or other drugs or may require a hysterectomy; techniques for local ablation, including high-intensity focused ultrasound and arterial embolization, continue to evolve.
Physical examination in patients with severe adenomyosis may reveal an enlarged but soft uterus.
Ultrasound of the uterus with adenomyosis may reveal the organ to be of normal size or enlarged. The echo texture of the affected regions is different from that of normal myometrium (Fig. 19.11); it may be heterogeneous or more echogenic than myometrium; hypoechoic myometrial striations have been described as well. There may be very small cysts in the abnormal regions, along with small echogenic nodules. The endometrial stripe may be widened and indistinct. If the abnormality is focal, it is important to try to distinguish it from a fibroid because the latter is more amenable to local resection. Fibroids are usually more crisply outlined, may have prominent peritumoral vessels, and are more likely to have large internal liquefied regions in areas of necrosis. Transvaginal ultrasound may approach the sensitivity of MRI in detecting adenomyosis; transabdominal ultrasound is not very sensitive for this disease.
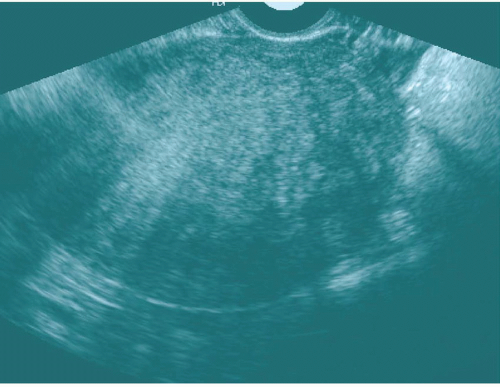 FIGURE 19.11. Adenomyosis. Transvaginal ultrasound shows an enlarged uterus with a finely heterogeneous echo pattern. (Courtesy of Sherelle Laifer-Narin, M.D.) |
MRI of adenomyosis may reveal the process to be focal or diffuse. The abnormal regions always involve the junctional zone; if the zone remains visible, it is almost certain to be thickened. Adenomyosis is often invisible on T1-weighted images, but occasionally, small hemorrhagic regions may appear bright. On T2-weighted images, the affected regions appear to be of lower intensity than normal myometrium, and there is usually fine heterogeneity (Fig. 19.12). Small regions of fluid or blood appear as bright speckles, which may be surrounded by thin dark rims. The margins of regions of adenomyosis are usually less well defined than those of fibroids.
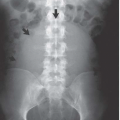
Hysterosalpingography may reveal regions of adenomyosis because the small glandular regions within it may fill with contrast and produce small collections in the regions of the myometrium (Fig. 19.13). Sonohysterography may reveal small saline-filled tracks from the endometrial cavity into the myometrium.
Hyperplasia
Hyperplasia of the endometrium may occur in premenopausal or postmenopausal women. It is almost always a response to hyperestrogenism, which in turn can be encountered in a variety of circumstances, including anovulatory states, estrogen-producing tumors, or exogenous administration of estrogen or tamoxifen. Most cases do not progress to endometrial carcinoma, but those that reveal severe cytologic atypia may do so.
Pathologically, the hyperplastic endometrium may exist as a smoothly thickened layer, a micronodular surface, or macroscopic
polyps; the hyperplastic tissue may contain cysts. Endometrial hyperplasia may produce irregular bleeding in premenopausal women and may cause postmenopausal bleeding.
polyps; the hyperplastic tissue may contain cysts. Endometrial hyperplasia may produce irregular bleeding in premenopausal women and may cause postmenopausal bleeding.
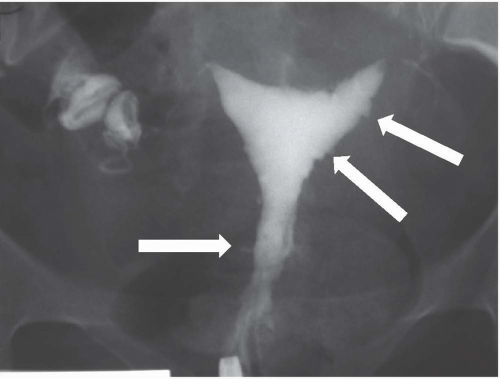 FIGURE 19.13. Adenomyosis. Hysterosalpingography reveals an irregular endometrial surface with small regions of penetration of contrast into the myometrium (arrows). |
Hysterosalpingography and saline infusion sonohysterography may reveal the endometrial surface to be irregular, nodular, or polypoid; the abnormalities may be focal or diffuse. In some cases, the appearance may overlap with that of endometrial carcinoma.
Ultrasound reveals various degrees of thickening of the endometrial stripe, especially if transvaginal ultrasound is used. Sonohysterography reveals a thickened ring of endometrial tissue around the saline-filled endometrial cavity. In premenopausal women, a fullthickness stripe greater than 14 mm in width may indicate endometrial hyperplasia or other pathology; in postmenopausal women, the stripe should be considered abnormal if it is greater than 8 mm in thickness (Figs. 19.14 and 19.15). High-quality transvaginal ultrasound is quite sensitive in detecting hyperplasia and cancer; in a postmenopausal woman, an endometrial stripe of less than 4 or 5 mm in thickness constitutes strong evidence that there is neither.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree