32 Duplex Ultrasound Evaluation of the Uterus and Ovaries
Duplex and color Doppler imaging have become a routine part of the ultrasound evaluation of the female pelvis.1 These techniques are utilized for both transabdominal and endovaginal examinations. The combination of Doppler ultrasound with endovaginal scanning is particularly valuable for gynecologic investigations because there is improved resolution and increased sensitivity to blood flow. The term endovaginal color flow Doppler imaging (EVCF) is used to describe this pairing of techniques.2 We routinely utilize Doppler ultrasound in a number of different applications, including the following:
1. Identification of the dominant follicle or corpus luteal cyst in patients with pelvic pain or suspected ectopic pregnancy
2. Detection of placental tissue in abnormal intrauterine pregnancy, ectopic pregnancy, and retained products of conception
3. Diagnosis of ovarian torsion
4. Characterization of ovarian and adnexal masses
5. Detection of a number of uterine abnormalities, including fibroids, polyps, and tumors, as well as vascular abnormalities such as arteriovenous malformation and the pelvic congestion syndrome.
Technical Issues
Because the technical aspects of color flow imaging are covered in Chapter 3, this chapter emphasizes just the key points pertinent to pelvic sonography. Similar to other color and pulsed Doppler examinations, Doppler evaluation of the uterus and ovaries should be considered a dynamic process, requiring adjustment of the color flow parameters according to the type of examination. Using the manufacturer’s settings (presets) is a good starting point for any examination. They serve as a general guide and can be adjusted to improve visualization of blood flow. Presets are helpful to novice or beginner sonographers and sonologists, particularly when the initial demonstration of color flow is suboptimal but critical for diagnosis.
It is important to remember that color, power, and pulsed Doppler images are based on the same physical principles but display different information.3 Color Doppler images are based on the mean velocity display of reflected frequency shifts. In other words, the frequencies reflected from the moving red blood cells are averaged over time and presented on the image. Color Doppler displays a range of velocities on the image but does not provide absolute or peak systolic velocity information. Pulsed Doppler is used to determine the peak velocity at a particular location.
Power (amplitude) Doppler images are determined by the strength or amplitude of the returning Doppler shifts.4 The frequency shifts are amplified and displayed along with the gray-scale information. Power Doppler provides three to five times increased sensitivity to blood flow, compared with color flow imaging. There is less dependence on the angle of insonation, so flow can be demonstrated at angles close to 90 degrees. Thus, very weak Doppler shifts are presented on the power Doppler image. Advantages of power Doppler include improved vascular detail, faster localization of blood flow for pulsed Doppler sampling, and assessment of global tissue perfusion.
These settings should be adjusted to improve the detection of color flow for each study, as they are fundamental to the detection of low-flow states.3 To detect low-velocity flow, we decrease the PRF, increase the color gain, and/or decrease the wall filter settings. When flow velocities are high and we want to reduce the degree of color noise or aliasing in the image, we increase the PRF or color velocity scale, decrease the color gain, and/or increase the wall filter. Experience and practice with different parameter settings will increase understanding of the interrelationships between these settings and lead to improved detection of flow. As always, the focal zone should be placed near the region of interest.
Normal Anatomy and Hemodynamics
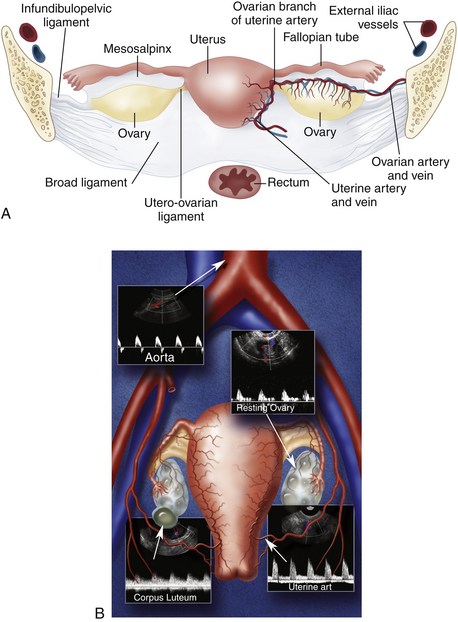
A thorough sonographic evaluation of the uterus and ovaries is usually performed in conjunction with Doppler assessment for blood flow. We usually begin our evaluation with the uterus and then turn our attention to the adnexa and evaluation of the ovaries. The uterus is a pear-shaped, midline structure that is usually easy to identify (Figure 32-1). Measurements of the length, width, and anteroposterior diameter of the uterus should be obtained. We also evaluate the thickness of the endometrium as well as the cervix and the presence and location of any uterine masses. The ovaries are variable in shape and location. Although the ovaries can be identified with either transabdominal or endovaginal scanning, a combination of techniques may be necessary for complete evaluation. The ovaries are also measured in three dimensions. The presence of cysts or masses is noted and correlated with the menstrual cycle.
Color and pulsed Doppler examination requires knowledge of the vascular anatomy and hemodynamic changes of the female pelvis. The vessels most frequently examined in the pelvis include the iliac, uterine, and ovarian arteries and veins (see Figure 32-1).5 These vessels may be identified with both transabdominal and endovaginal imaging. EVCF affords improved resolution and vascular detail compared with the transabdominal approach.
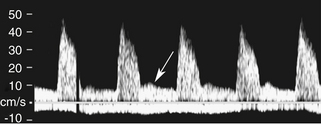
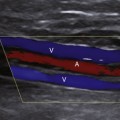
The uterine artery is a branch of the internal iliac artery and penetrates the uterus at the lower uterine segment (see Figure 32-1, B). Uterine artery branches course toward the uterine fundus and cervix as well as toward the ovaries in the broad ligament. Color and pulsed Doppler sampling of the uterine artery reveals high-impedance, low diastolic flow in the nongravid state.5 A characteristic diastolic “notch” is usually noted (Figure 32-2). Identification of the notch is helpful in characterizing waveforms found in the uterus and adnexa as originating from the uterine artery. There is a gradual decrease in resistance to flow in the spectral samples obtained from the uterine arteries during the second trimester of pregnancy. The decrease in resistance and resistivity index is related to increased blood flow, particularly in diastole, necessary for normal placental and fetal growth. Continuous low-resistance blood flow should be detected in the placenta and umbilical arteries supplying the fetus.
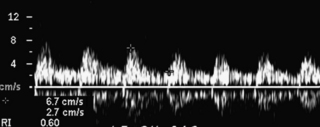
Each ovary receives a dual blood supply, as shown in Figure 32-1, A. The ovarian artery originates from the abdominal aorta and descends to the pelvis. The ovary also receives branches from the uterine artery that course along the broad ligament. Blood flow patterns observed during color and pulsed Doppler sampling of the ovary vary depending on the phase of the ovulatory cycle. Low-velocity, high-impedance waveforms are usually noted early in menses into the follicular phase. (Figure 32-3). This is seen during the first menstrual week, when the ovaries are dormant and before the formation of the dominant follicle and corpus luteal cyst.6
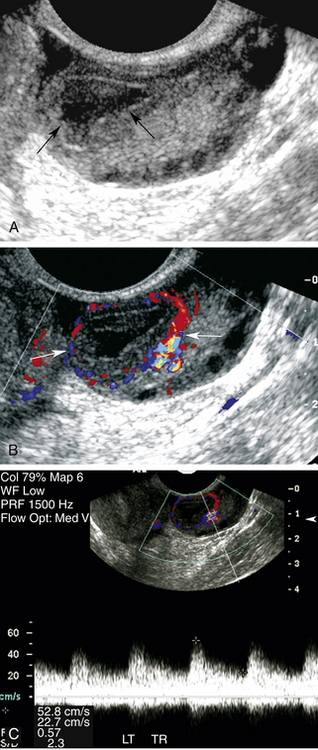
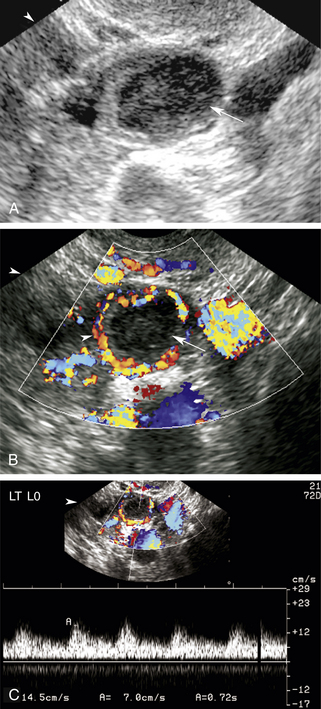
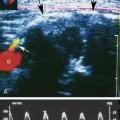
The luteal phase coincides with the extrusion of the mature egg or oocyte and formation of the corpus luteal cyst. Thickening of the cyst walls is seen with gray-scale imaging. Color Doppler demonstrates a ring of vascularity (“ring of fire” pattern) around the luteal cyst, related to formation of tiny vessels in the walls of the cyst.7 Pulsed Doppler shows a marked increase in peak systolic and end-diastolic velocities (Figure 32-4). The increased velocities are related to neovascularization of the corpus luteum, required for oocyte maturation and hormonal activity.8
I originally described the “ring of fire” color flow pattern to represent the increased vascularity noted around the periphery of an extrauterine gestational sac.9 Subsequently, it became very clear that a similar pattern of peripheral blood flow occurs with the formation of a corpus luteal cyst. In fact, we look for the ring of increased vascularity in the ovary to locate and characterize luteal cysts. It should be clear that the “ring of fire” color flow pattern alone cannot distinguish between ectopic pregnancies and luteal cysts. Investigators have tried to identify discriminating Doppler parameters to distinguish luteal from ectopic flow.10,11 This is difficult due to overlap in peak systolic velocity and resistivity index measurements between luteal cysts and ectopic pregnancies. The origin of the Doppler signal, from within the ovary or from an adnexal mass, allows more accurate characterization of a corpus luteal cyst or ectopic pregnancy than velocity or resistivity index alone. Therefore, we do not use Doppler to distinguish luteal from ectopic flow but to localize the site of origin of the signals to determine their significance.
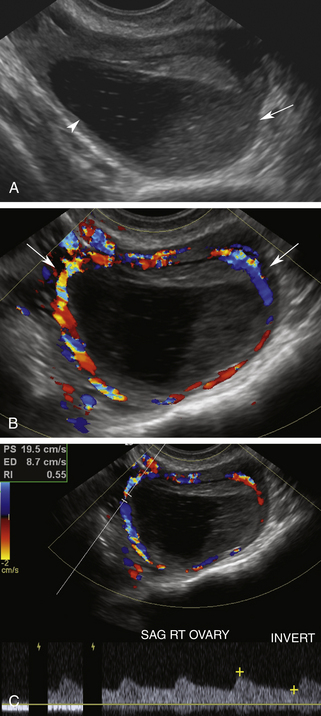
Color and pulsed Doppler signals obtained from a postmenopausal ovary have low peak systolic velocities similar to ovaries in the follicular phase7 (Figure 32-5). This is typical of ovaries in the resting state. Because postmenopausal ovarian vessels carry low-velocity flow, this vascularity may be very difficult to visualize with conventional color Doppler flow settings. Low color velocity scale (PRF) and color wall filter adjustments may be necessary to detect postmenopausal ovarian blood flow. Power Doppler imaging improves the visualization of ovarian flow, particularly in postmenopausal women. Because postmenopausal ovaries no longer ovulate, they remain relatively quiescent and are associated with little or no diastolic flow.
Current Applications
Current applications of transabdominal scanning and EVCF include identification of the corpus luteal cyst, detection of intrauterine placental flow, diagnosis of ectopic pregnancy and retained products of conception, evaluation for ovarian torsion, and characterization of adnexal masses and uterine abnormalities.
Corpus Luteal Cyst
Identification of the dominant follicle or corpus luteal cyst has proven extremely helpful in patients who present with pelvic pain, adnexal mass, or ectopic pregnancy. Ovarian cysts are the most common cause of acute pelvic pain in premenopausal patients.12 The pain is usually associated with enlargement of the cyst during the midportion of the menstrual cycle and precedes cyst rupture and the release of fluid.
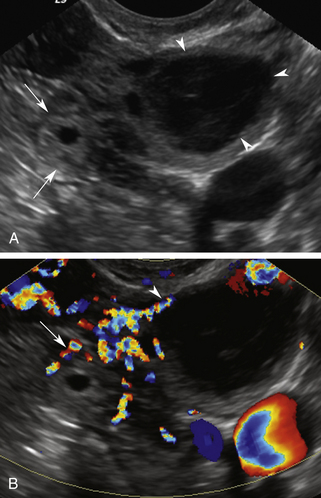
Color and pulsed Doppler are important tools for the characterization of complex ovarian cysts. Color Doppler can demonstrate the ring of increased vascularity in the wall of the corpus luteal cyst (Figure 32-6). As mentioned earlier, the peripheral vascularity is related to neovascularization associated with the formation of the corpus luteal cyst.8 The increased blood flow identified with color Doppler is associated with elevated peak systolic velocities and low-resistance flow. Dillon and colleagues13 demonstrated a peak systolic velocity of 27 ± 10 cm/sec and a resistivity index of 0.44 ± 0.09 for corpus luteal cysts. The demonstration of peripheral vascularity with color or power Doppler increases the conspicuity of the corpus luteal cyst, even when it is filled with blood and is isoechoic to ovarian tissue on gray-scale images. The lack of vascularity within the central part of the lesion suggests it is a hemorrhagic cyst.12 This is particularly helpful when gray-scale evaluation demonstrates wall thickening, nodules, or septations within the cyst cavity. The absence of flow within the cyst cavity suggests that any solid material within the cyst is likely related to hematoma or retracting clot and not tumor (Figure 32-7). A follow-up ultrasound study 6 to 8 weeks later, during the first week of a subsequent menstrual cycle, is recommended to assess for complete resolution of the complex cyst and to exclude the possibility of tumor.
The recognition of the corpus luteal cyst also aids in the diagnosis of ectopic pregnancy. Approximately 85% to 90% of ectopic pregnancies occur on the same side as the corpus luteal cyst.9 Identification of the corpus luteum determines the side of ovulation and directs the examiner to the expected site of the ectopic pregnancy (Figure 32-8). Color and pulsed Doppler also play a role in the identification and follow-up of ectopic pregnancy, which is our next topic of discussion.
Ectopic Pregnancy
Ectopic pregnancy occurs in approximately 2% of pregnancies and is the leading cause of pregnancy-related deaths during the first trimester.14 There is a rising incidence of ectopic pregnancy that is related to the following:
An increased number of patients at risk
New techniques that allow earlier diagnosis
Improved treatment for salpingitis and ectopic pregnancy
Increased utilization of ovulation induction and assisted reproduction techniques
It is important to understand and solicit risk factors from patients with suspected ectopic pregnancy.15 Any process that produces scarring or obstruction of the fallopian tube predisposes to ectopic pregnancy. The obstruction may be related to prior pelvic surgery or tubal ligation, prior ectopic pregnancy, or history of pelvic inflammatory disease or salpingitis. The use of an intrauterine contraceptive device or the “morning after” pill may also increase the risk for ectopic pregnancy. In vitro fertilization also increases the rate of ectopic pregnancy because of multiple risk factors, including infertility, ovulation induction, and embryo transfer with retrograde migration of the embryo into the fallopian tube. Infertility is associated with multiple anatomic and physiologic conditions that increase risk for ectopic pregnancy. Other risk factors include prior cesarean section, in utero exposure to diethylstilbestrol, and sterilization.
The clinical presentation of ectopic pregnancy is variable; however, a positive pregnancy test, pelvic pain, an adnexal mass, and/or vaginal bleeding raise clinical suspicion for this condition. The classic triad of pelvic pain, adnexal mass, and vaginal bleeding occurs only in approximately 45% of patients.16 Patients may be asymptomatic or have focal or generalized pelvic or abdominal pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree