Key points
- •
Intracranial dural arteriovenous fistulae (DAVF) are pathologic shunts between dural arteries to dural veins or a venous sinus and are an important cause of pulsatile tinnitus.
- •
Digital subtraction angiography allows the accurate characterization and classification of DAVF and remains the gold standard modality for their diagnosis.
- •
The pattern of venous drainage determines the type and risk of intracranial bleeding of DAVF.
- •
The goal of treatment is to obliterate the site of the shunt with elimination of retrograde cortical venous drainage.
- •
Endovascular approach has become a first-line option for treatment of DAVF.
Introduction
Dural arteriovenous fistulae (DAVF) are abnormal connections between meningeal arteries and dural venous sinuses, meningeal veins, or cortical veins. By definition, DAVF are located within the dura, most frequently on the wall of or immediately around the venous sinuses. They account for approximately 10% to 15% of intracranial arteriovenous shunts and are typically encountered in middle-aged adults with a median age of onset in the sixth decade.
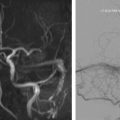
Most reported intracranial DAVF involve the transverse and sigmoid sinuses. Patients with DAVF involving the transverse and sigmoid sinuses ( Fig. 1 ) often present with ipsilateral retroauricular pain and pulsatile tinnitus that is usually audible on auscultation of the mastoid area. Pulsatile tinnitus is known to result from nonlaminar blood flow caused by increased blood flow or a reduced vascular cross-sectional area, and is often secondary to an identifiable, vascular anomaly. This symptom is associated with approximately 80% of transverse and sigmoid sinus DAVF, which could be explained by direct transmission of the venous bruit to the inner ear through the temporal bone. In addition, intracranial DAVF are the most common cause of objective pulsatile tinnitus in patients with normal otoscopic examination.
Pulsatile tinnitus is often the only presenting symptom in low-grade DAVF with solely anterograde venous drainage (Borden type I, discussed later). The pulsatile tinnitus caused by DAVF can be unbearably loud and can often be heard by the clinician too. The occipital artery often contributes to the blood supply of these lesions and is generally hypertrophied. Compression of the occipital artery against the mastoid process therefore reduces the tinnitus on physical examination.
The causes of DAVF remain incompletely understood. Although in the pediatric population most DAVF are congenital and often associated with structural venous abnormalities, most DAVF are thought to be acquired. The development of venous or dural sinus thrombosis and venous hypertension with aberrant angiogenesis has been associated with the pathogenesis of these lesions. The causal role of venous thrombosis is supported by the association of DAVF with hypercoagulable states such as factor V Leiden; hyperhomocysteinemia; and antithrombin, protein C, and protein S deficiencies. The altered angiogenesis leading to DAVF can occur within the dura following an inciting event such as trauma, surgery, chronic infection, or sinus thrombosis. However, many cases of DAVF are sporadic without any clear inciting event.
The natural history of DAVF varies from spontaneous resolution to fatal hemorrhage, and this depends to a great extent on their venous drainage patterns. Given this large spectrum, it is not the diagnosis but the expected prognosis of the disease that indicates treatment, which makes it mandatory to obtain a proper classification of patients by diagnostic angiography.
Introduction
Dural arteriovenous fistulae (DAVF) are abnormal connections between meningeal arteries and dural venous sinuses, meningeal veins, or cortical veins. By definition, DAVF are located within the dura, most frequently on the wall of or immediately around the venous sinuses. They account for approximately 10% to 15% of intracranial arteriovenous shunts and are typically encountered in middle-aged adults with a median age of onset in the sixth decade.
Most reported intracranial DAVF involve the transverse and sigmoid sinuses. Patients with DAVF involving the transverse and sigmoid sinuses ( Fig. 1 ) often present with ipsilateral retroauricular pain and pulsatile tinnitus that is usually audible on auscultation of the mastoid area. Pulsatile tinnitus is known to result from nonlaminar blood flow caused by increased blood flow or a reduced vascular cross-sectional area, and is often secondary to an identifiable, vascular anomaly. This symptom is associated with approximately 80% of transverse and sigmoid sinus DAVF, which could be explained by direct transmission of the venous bruit to the inner ear through the temporal bone. In addition, intracranial DAVF are the most common cause of objective pulsatile tinnitus in patients with normal otoscopic examination.
Pulsatile tinnitus is often the only presenting symptom in low-grade DAVF with solely anterograde venous drainage (Borden type I, discussed later). The pulsatile tinnitus caused by DAVF can be unbearably loud and can often be heard by the clinician too. The occipital artery often contributes to the blood supply of these lesions and is generally hypertrophied. Compression of the occipital artery against the mastoid process therefore reduces the tinnitus on physical examination.
The causes of DAVF remain incompletely understood. Although in the pediatric population most DAVF are congenital and often associated with structural venous abnormalities, most DAVF are thought to be acquired. The development of venous or dural sinus thrombosis and venous hypertension with aberrant angiogenesis has been associated with the pathogenesis of these lesions. The causal role of venous thrombosis is supported by the association of DAVF with hypercoagulable states such as factor V Leiden; hyperhomocysteinemia; and antithrombin, protein C, and protein S deficiencies. The altered angiogenesis leading to DAVF can occur within the dura following an inciting event such as trauma, surgery, chronic infection, or sinus thrombosis. However, many cases of DAVF are sporadic without any clear inciting event.
The natural history of DAVF varies from spontaneous resolution to fatal hemorrhage, and this depends to a great extent on their venous drainage patterns. Given this large spectrum, it is not the diagnosis but the expected prognosis of the disease that indicates treatment, which makes it mandatory to obtain a proper classification of patients by diagnostic angiography.
Classification
Several classification schemes have been proposed from different aspects of DAVF to grade their risk and natural course. The classifications put forward by Cognard and colleagues and Borden and colleagues are currently the most widely used ( Box 1 ). Both are used in everyday clinical practice and emphasize the venous drainage patterns associated with the fistula. The 3-step Borden classification is simple to apply and categorizes DAVF based on the site of venous drainage (dural sinus vs cortical vein) as well as the absence or presence of cortical venous drainage (CVD). The Cognard classification is more complex and incorporates additional information on the flow direction in the dural sinus (antegrade vs retrograde) as well as the presence or absence of venous ectasia in the involved cortical veins.
Borden Classification
I: Venous drainage into dural sinus
II: Venous drainage into dural sinus with cortical venous drainage (CVD)
III: Venous drainage directly into subarachnoid vein (CVD only)
Cognard Classification
I: Venous drainage into dural sinus with normal antegrade flow
IIa: Venous drainage into dural sinus with retrograde flow
IIb: Venous drainage into dural sinus with normal antegrade flow and CVD
IIa + b: Venous drainage into dural sinus with retrograde flow and CVD
III: Venous drainage directly into subarachnoid vein (CVD only)
IV: Venous drainage directly into subarachnoid vein with venous ectasia
V: Venous drainage directly into spinal perimedullary veins
Both grading systems highlight the importance of CVD and associated venous drainage pattern to risk of hemorrhage and neurologic deficit. The presence of CVD (Borden type II and III, Cognard types IIb–V) is an aggressive feature that places DAVF in a higher risk category. The higher the type in either classification system, the more likely the DAVF is to be symptomatic as a result of venous congestion. In addition, it has been suggested that subdividing lesions with CVD into symptomatic and asymptomatic types may further improve the accuracy of risk stratification, with symptomatic lesions showing significantly higher risk of annual hemorrhage than asymptomatic types.
Although lesions with aggressive features can occur in any intracranial location, the anterior cranial fossa and tentorium seem to have a higher incidence of aggressive lesions, likely because of the higher likelihood of development of CVD caused by the lack of adjacent dural sinuses to provide venous drainage at these sites. Davies and colleagues reported that 69% of hemorrhagic lesions in their series of 102 DAVF occurred either in the anterior cranial fossa or the tentorium, which was a disproportionately high rate given that these two locations accounted for only 18% of the lesions in their study. Other studies have also corroborated these findings.
Imaging findings
Given their wide range of clinical presentations and the lack of specificity of symptoms, the diagnosis of DAVF can be challenging. Nonenhanced computed tomography (CT) and conventional magnetic resonance (MR) imaging can often appear unremarkable with benign DAVF. When symptomatic, the 2 most common presentations of DAVF are intracranial hemorrhage and nonhemorrhagic neurologic manifestations. In either case, diagnostic evaluation usually starts with noncontrast head CT and MR imaging.
The patterns of blood distribution detected with noncontrast head CT or MR imaging in patients with intracranial hemorrhage from ruptured DAVF are not specific. The most common pattern of intracranial hemorrhage in ruptured DAVF is intraparenchymal, usually lobar. Subarachnoid hemorrhage (SAH) is less commonly seen, and is most often associated with DAVF with leptomeningeal or pure CVD. Cognard type V has a high propensity for presenting with SAH. Intraventricular hemorrhage may be present as a result of extension of intraparenchymal or subarachnoid hemorrhage. Subdural hematomas are rarely seen, but have been reported in anterior cranial fossa DAVF.
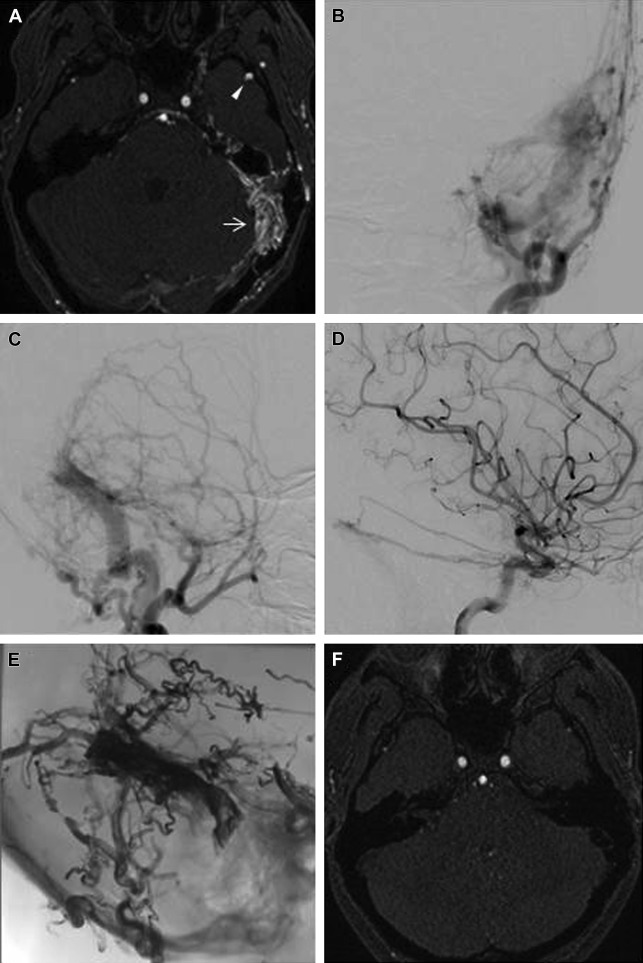
A focused approach in relation to the patient’s symptoms and the possible location of the lesion is required when interpreting cross-sectional studies in patients with suspicion of DAVF. With larger fistulae, CT or MR can show prominent vessels or flow voids that may be associated with a dural sinus ( Fig. 2 ). Cross-sectional imaging may also reveal engorged orbital veins and proptosis in cases of cavernous sinus DAVF, and intraosseous serpentine flow voids can be appreciated in anterior condylar DAVF. In addition, MR imaging can provide complementary information that can help evaluate for associated hydrocephalus, other possible causes of pulsatile tinnitus, as well as signal abnormalities secondary to intracranial venous hypertension.
CT angiography (CTA) and MR angiography (MRA) may detect the fistula and can be used to screen patients suspected of harboring DAVF (see Fig. 2 ). The fistula may appear as prominent vessels associated with the meninges or dural sinus wall. Enlarged feeding arteries, early dural sinus opacification, and prominent draining veins can all be well characterized with CTA and MRA with very good accuracy.
Recent advances in imaging technology have allowed the development of time-resolved MRA and CTA, which use the first-pass effect of intravenous contrast to evaluate the dynamics of blood flow in the intracranial circulation. This technique allows the differentiation of arterial and venous phases and permits the better visualization of arteriovenous shunting. Time-resolved MRA at 3T has been reported to have a sensitivity and specificity of 100% compared with angiography for the screening, the follow-up, and the posttreatment evaluation of intracranial DAVF. Time-resolved CTA has also been found to be almost equivalent to angiography in diagnosing and classifying intracranial DAVF.
Digital subtraction angiography remains the gold standard to diagnose DAVF. Thanks to its superior spatial and temporal resolution, catheter angiography can clearly characterize both the arterial supply and venous drainage of the fistula, as well as identify important features of the fistula, such as presence of CVD, venous outflow obstruction, and arterial pedicle or venous aneurysms. In addition, catheter angiography is essential for treatment planning, for which a thorough evaluation of all possible arterial feeders and careful depiction of normal and abnormal veins are required.
General management
Conservative treatment is often a consideration for patients with low-grade, benign fistulas (Borden I; Cognard I, IIa). Spontaneous thrombosis of DAVF may occasionally occur, more commonly in slow-flow cavernous sinus lesions. Carotid self-compression may help promote resolution of the fistula in a minority of these cases. However, patients with benign, low-grade lesions electing conservative management should undergo clinical and imaging follow-up because of the risk of conversion to an aggressive lesion.
In contrast, high-grade, aggressive lesions carry significant mortalities as well as important risk of bleeding and nonhemorrhagic neurologic deficits. Variceal or aneurysmal venous dilatation, leptomeningeal venous drainage, and galenic drainage are significant factors predisposing patients to an aggressive neurologic course. The overall rate of hemorrhage from DAVF has been estimated at approximately 1.8%. In patients initially presenting with hemorrhage, the risk of rebleeding within the first 2 weeks following the initial hemorrhage has been estimated at up to 35%. Therefore, aggressive DAVF should be treated early to avoid complications. In addition, in low-grade lesions with severe debilitating symptoms, including debilitating pulsatile tinnitus, treatment should be considered to reduce symptoms.
At present available therapeutic options for intracranial DAVF include no treatment, conservative management, endovascular treatment, surgery, a combination of endovascular treatment and surgery, and radiosurgery. In the past, DAVF were treated with surgical disconnection of the pathologic arteriovenous communication and possible resection of the involved segment of the dural sinus. However, with newer endovascular therapies and newer, more navigable microcatheters available, endovascular embolization has become the first-line treatment of DAVF. Surgery is used either in combination with the endovascular techniques or when the endovascular technique fails.
Endovascular treatment
The goal of endovascular therapy is the elimination of the arteriovenous shunt. Note that the pathologic entity of DAVF seems to be located within the wall of dural sinuses, veins, or leptomeningeal veins. The pathophysiologic effect of the shunt is exercised on the venous system. Complete and permanent cure can be achieved only by obliterating all pathologic connections between the arterial and venous side of the lesion. This outcome can be obtained by approaching the site of the shunt through the feeding arteries or by sealing the lumen of the draining venous structures. Partial embolization may temporarily alleviate symptoms, but is unlikely to result in a long-term cure. In addition, partial occlusion could negatively alter the venous drainage pattern, potentially inducing CVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree