Abstract
Fetal echogenic bowel (FEB) is often a benign finding during second trimester ultrasound. It is defined as one or more areas of bowel that are as bright as nearby bone on the lowest gain setting. The presence of echogenic bowel has been associated with increased risk for aneuploidy, fetal cystic fibrosis, bowel obstruction, and infectious exposures. FEB has additionally been associated with bleeding in pregnancy, as well as abnormalities of fetal growth. If diagnosed, the presence of FEB should prompt consideration for infectious and genetic testing. The pregnancy should be followed with serial ultrasound to assess both fetal growth and development of further gastrointestinal abnormalities. However, most infants with prenatally diagnosed FEB go on to have uncomplicated neonatal courses.
Keywords
echogenic bowel, soft marker, GI abnormalities
Introduction
Fetal echogenic bowel (FEB) is typically seen during second trimester prenatal ultrasound. FEB occurs when the fetal bowel appears with the same or greater echogenicity than do surrounding bony structures. When seen in the second trimester, FEB has been associated with increased risk for fetal cystic fibrosis, aneuploidy, gastrointestinal abnormalities, growth restriction, and viral infections. However, it is important to realize that the majority of patients with isolated FEB will have no associated adverse outcomes.
Disorder
Definition
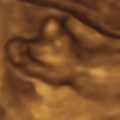
While there is no standard definition for FEB, most practitioners use bowel that appears as bright as bone when minimal gain is used. Typically, the iliac wing is used as the bony reference point. The area may be singular or multifocal within the fetal abdomen, and should be homogeneously hyperechoic. Echogenic areas that create distal shadowing are suggestive of calcification. It is important to recognize that high frequency transducers can create the appearance of echogenicity, so consider using a lower frequency (e.g., 5 MHz) transducer to avoid overdiagnosis. Furthermore, newer machines with improved penetration often lead to an increase in the diagnosis of echogenic lesions. Similarly, women with lower body mass index (BMI) appear to be more often diagnosed with echogenic bowel. Therefore, care should be taken to adjust the gain to avoid overdiagnosis.
Prevalence and Epidemiology
The reported prevalence of FEB ranges from 0.2% to 1.4% during the second trimester.
Etiology and Pathophysiology
FEB is a nonspecific ultrasound finding that confers an increased risk for a range of pathologies. In many cases FEB is considered a normal variant, especially when isolated; however, one-third of cases may have a pathologic cause. Each pathologic entity has differing theories of the pathophysiology resulting in FEB, which will be addressed subsequently.
Bleeding
A bleeding event during pregnancy may result in blood in the amniotic cavity. The fetus then swallows and digests the blood, resulting in deposition of blood components into the gastrointestinal system. As blood is echogenic, the presence of blood in the fetal gastrointestinal system can increase its echogenicity. FEB can persist beyond 2 weeks after a bleeding event, therefore assessing the patient’s recent history is important. Additional findings more consistent with a bleeding cause would be the presence of echogenic material in the fetal stomach or visualization of a subchorionic fluid collection.
Aneuploidy
FEB is considered a soft marker for fetal aneuploidy, most commonly trisomy 21. In some studies it increases the likelihood for trisomy 21 by 6.1-fold. FEB has been associated with other aneuploidies as well, with an overall aneuploidy risk of 6.7% in isolated FEB. In one retrospective cohort, the presence of FEB, when combined with other soft markers and major structural abnormalities, increased the chromosomal abnormality rate to 7.7% and 17.4%, respectively. The pathophysiology of FEB in aneuploidy is unclear. Fetuses with genetic abnormalities may have gastrointestinal complications, such as decreased intestinal motility or malformation. The likelihood ratio of 6.1 can be used to recalculate a patient’s aneuploidy risk from their a priori age-related risk or from the results of analyte screening. It is unclear whether the results of cell-free DNA (cfDNA) screening should be adjusted based on the finding of FEB.
Cystic Fibrosis
There is a well-established correlation between FEB and fetal cystic fibrosis. Cystic fibrosis, a malfunction of an important chloride channel, results in pancreatic insufficiency and hypomotility. This leads to increased secretions and thicker meconium, which is thought to be the etiology for FEB in these patients. In a large French cohort, FEB was seen in 10% of infants with genetic diagnosis of cystic fibrosis. Conversely, 3% of all fetuses with FEB had cystic fibrosis. This incidence is much higher than the expected incidence of 1 : 2000–1 : 3000 live births in a Caucasian population.
Growth Restriction
FEB has been associated with fetal growth restriction. The etiology is unknown, but has been postulated to be related to reduced blood flow to the intestine with preferential shunting to vital organs such as the brain. However, one study indicated the opposite, demonstrating vasodilation in the superior mesenteric and celiac trunks in growth-restricted fetuses. Fourteen percent to 23% of fetuses with FEB are also growth restricted. The presence of growth restriction and FEB increases the risk for fetal demise. This risk is further increased by the presence of elevated maternal alpha-fetoprotein and/or oligohydramnios.
Digestive Tract Malformations
Disorders of the gastrointestinal system have been associated with the presence of FEB. FEB may be one of the earliest signs of small or large bowel obstruction. The etiology behind this is that decreased transit time allows for increased fluid reabsorption, resulting in increased density of intestinal contents. With continued observation, more characteristic findings of bowel obstruction, such as dilation, become apparent. Additional disorders of the gastrointestinal system reported in case reports/series associated with FEB include imperforate anus, intestinal atresias, volvulus, Hirschprung disease, and Zellweger Syndrome.
Infection
Congenital infection may result in FEB, possibly because of direct bowel damage. The most common prenatal infection related to FEB is cytomegalovirus (CMV). It is estimated that 3% of fetuses with FEB have CMV infection. However, this association is controversial, as it has not been demonstrated in all studies. Other potential infectious etiologies include toxoplasmosis, varicella, herpes, and parvovirus.
Manifestations of Disease
Clinical Presentation
FEB is typically seen at a second trimester ultrasound and is considered a sign of potential pathologic conditions. In a large cohort study, FEB was associated with chromosomal abnormalities in 4.3% of cases, cystic fibrosis in 3.1%, digestive tract abnormalities in 2.9%, infectious disease 2.8%, intrauterine growth restriction in 2.3% and fetal death in 1.9%. A risk of poor perinatal outcome of 33% was demonstrated in one large series.
After birth, the majority of neonates with prenatal isolated FEB have no major sequelae. Interestingly, only 11% of children were identified as having any intestinal symptoms of constipation, chronic abdominal pain, milk intolerance, or reflux after having FEB.
Imaging Technique and Findings
Ultrasound.
FEB is suspected during ultrasound evaluation of the fetal abdomen. If one or more areas appear particularly echogenic, the gain should be reduced until nearby bone is nearly visible ( Figs. 22.1 and 22.2 ). If the area in question is still visible, either as bright or brighter than the bone, FEB is diagnosed. Typically, the iliac wing is used as the bony reference. There are no diagnostic requirements related to size, number, or shape of the echogenicities.