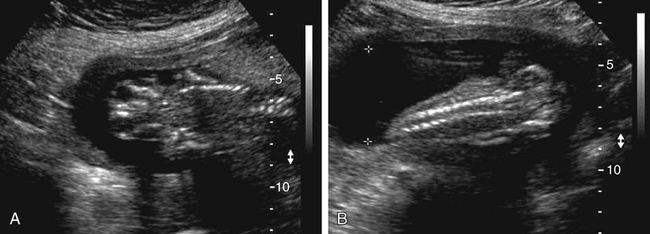
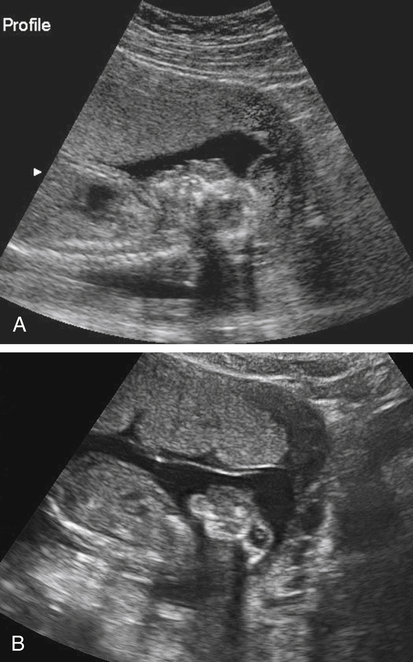
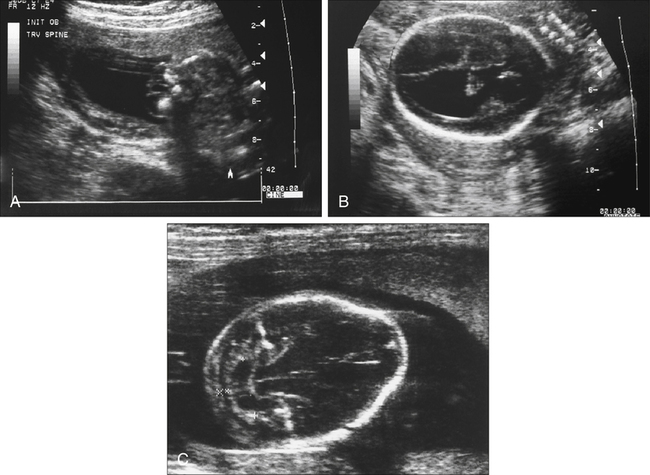
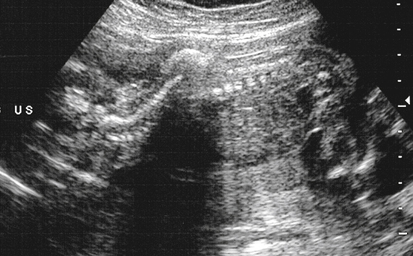
Armando Fuentes and Charlotte Henningsen • Describe the clinical use of serum alpha fetoprotein as a screen for various fetal anomalies. • Describe the appropriate diagnostic approach to a patient with an elevated alpha fetoprotein value. • Identify potential pregnancy complications in the setting of an unexplained elevated alpha fetoprotein value. • Identify the sonographic appearance of neural tube disorders commonly associated with an elevated alpha fetoprotein value. • Identify fetal abdominal wall anomalies associated with elevated alpha fetoprotein values. • Describe the sonographic appearance of abdominal wall defects commonly associated with an elevated alpha fetoprotein value. • Differentiate sonographic features of limb–body wall complex and amniotic band syndrome. Several studies have demonstrated the reliability of MSAFP screening for NTDs. Wang et al.1 published a meta-analysis comprising 684,112 patients screened in the second trimester that showed a sensitivity of 75.1 and specificity of 97.7. Detection of anencephaly is even higher. Data from various studies indicate that major fetal anomalies are present in 30% to 58% of patients with MSAFP levels of 5.0 MoM or greater.2,3 When a cutoff of 2.5 MoM is used, MSAFP screening detects approximately 88% of anencephalic fetuses and 79% of fetuses with open spina bifida. When no explanation can be determined for an elevated alpha fetoprotein value, despite sonographic and chromosomal investigation, 20% to 38% of pregnancies have adverse outcomes. These adverse outcomes include low birth weight, prematurity, intrauterine growth restriction, preeclampsia, and placental abruption.4 Sonography has become a very effective tool for detecting NTDs, and it has replaced MSAFP testing as a screening tool in some centers.5 Sonographic detection of NTDs is influenced by gestational age, maternal body habitus, and type of NTD. First-trimester screenings have reported greater than 90% detection rates for anencephaly and 80% detection rates for encephalocele. In the second trimester, sonographic screening has a detection rate of 92% to 95% for spina bifida.6 Failure of neural tube closure between the third and fourth week of embryologic development results in NTDs. Approximately 18 days after conception, the neural plate folds to form a central neural groove and bilateral neural folds. The neural folds fuse in the midline and begin to form the neural tube. Fusion progresses simultaneously toward the cranial and caudal ends. Closure of the anterior neuropore occurs by day 24, and closure of the posterior neuropore occurs by day 26. The cranial end of the neural tube becomes the forebrain, midbrain, and hindbrain, and failure of closure results in anencephaly. The caudal end of the neural tube becomes the spinal cord, and failure of posterior neuropore closure results in spina bifida.7 Most NTDs are either isolated defects or multifactorial. Several factors have been implicated, including folic acid deficiency,8 medications (valproic acid), genetic factors (MTHFR polymorphism, Meckel-Gruber syndrome),9 and vitamin B12 deficiency.10 Environmental factors associated with NTDs include hyperthermia, maternal diabetes mellitus, and obesity.11,12 The incidence of NTDs in the United States is commonly quoted as 1:1000; this is highly dependent on ethnic and geographic variability. The incidence appears to be higher in Hispanic and non-Hispanic whites compared with African Americans and Asians. Higher rates of NTDs have also been seen in the eastern and southern United States compared with the western United States. Additionally, there is an increased risk of recurrence in families with a history of a prior NTD. The introduction of screening programs and folic acid supplementation has significantly reduced the incidence of NTDs. In 2006, birth data from the United States revealed a combined incidence of anencephaly and spina bifida of 0.3:1000 live births, which is significantly lower than 1:1000 reported in the 1990s.13 Anencephaly, which results in absence of the cranium and brain above the orbits, is the most common NTD. It can also occur as a result of destruction of brain matter exposed to amniotic fluid in fetuses with acrania. It has been identified in 1:1000 pregnancies,14 but the incidence of live births is significantly lower. Anencephaly is diagnosed when absence of the calvaria above the orbits and absence of the brain are noted (Fig. 23-2, A). It is best seen in the coronal plane where the orbits appear prominent revealing a “frog’s eye” appearance (Fig. 23-2, B). There may be a small, abnormal remnant of tissue seen above the orbits, known as the cerebrovasculosa. If the fetal head is difficult to visualize because of a low vertex position, an endovaginal sonogram may be necessary. Polyhydramnios is commonly identified in gestations with anencephaly secondary to a decreased swallowing reflex. Complete evaluation of the spine involves viewing of spinous structures in the longitudinal and transverse aspects, from the nuchal origin to the distal sacrum. In the transverse plane, the posterior ossification centers tilt toward the midline. Spina bifida should be considered when the posterior ossification centers assume a splayed “V,” “C,” or “U” configuration when viewed in the transverse plane (Fig. 23-3, A). In addition, abnormal curvature of the spine (kyphosis, lordosis, or scoliosis) may be observed. The most superior aspect of vertebral misalignment defines the level of the lesion, which relates to outcome (Fig. 23-4); three-dimensional reconstruction may be useful in clarifying the anatomic level of the defect.15 An intracranial anomaly known as Arnold-Chiari malformation often accompanies spina bifida aperta. Arnold-Chiari malformation is characterized by cerebral ventriculomegaly (lateral ventricle >10 mm), decreased intracranial pressure leading to frontal bone collapse (lemon sign), abnormal curvature of the cerebellum as it is impacted into the posterior fossa (banana sign), decreased cerebellar size (which may lead to failure of identification), and obliteration of the cisterna magna (see Fig. 23-3, B and C). Cephalocele or encephalocele is defined as a defect in the cranium through which intracranial tissue protrudes, including meninges with or without brain tissue. The incidence is 0.8:10,000 to 4:10,000 live births.16 Occipital encephaloceles are usually considered within the NTD group. Associated conditions with occipital encephaloceles include Meckel-Gruber syndrome, amniotic band syndrome (ABS), cerebellar dysgenesis, short limb dysplasia, and warfarin syndrome. Frontal or parietal encephaloceles are not considered a NTD and may be environmental in origin. Sonographically, a cephalocele appears as herniation of the meninges with or without brain through a defect in the calvaria (Fig. 23-5). The sac may appear cystic or solid and can vary in size. Ventriculomegaly is often seen, and traction on the intracranial contents may result in microcephaly. Cephaloceles may also vary in appearance throughout gestation.
Elevated Alpha Fetoprotein
Maternal Serum Alpha Fetoprotein
Neural Tube Defects
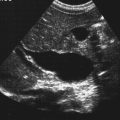
Anencephaly
Sonographic Findings
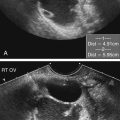
Spina Bifida
Sonographic Findings
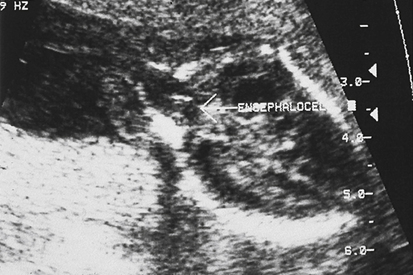
Cephalocele
Sonographic Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Elevated Alpha Fetoprotein