Following the introduction of endovascular techniques to treat extracranial carotid disease, the seminal challenge was to compete effectively with the tried and tested surgical alternative, carotid endarterectomy (CEA), which had largely already undergone and completed refinements in technique that improved clinical outcomes.1,2 Perhaps the biggest challenge was the effective control of the embolic burden of carotid artery stenting (CAS). It is known that periprocedural thromboembolic events occur frequently as a result of manipulation of guidewires, balloons, and stents.3 The consequences and clinical manifestations of these emboli are dependent on both size and number of emboli, on the temporal pattern of embolization, and on target organ sensitivity to emboli and ischemia. In the carotid territory, distal emboli may result in cerebral ischemia, stroke, and in the longer term, possibly cognitive decline and dementia, although the supporting evidence for this contention is not derived from the clinical setting of CAS.4 In the lower limb, distal embolization can result in critical limb ischemia and amputation through loss of runoff vessels. Use of embolic protection devices (EPDs) is most widely studied in the carotid circulation, and many of the device innovations and advances relate to CAS. Although there are no randomized trial data based on clinical outcomes to advocate their use, each type of device has been shown to capture macroemboli, implying additional protection of the brain during CAS when they are used. A systematic review reported that the relative risk of all stroke (0.59 in 24 studies, with concurrently reported protected and unprotected data) was significantly lower (I < 0.05) for protected than unprotected series. Additionally, the relative risk of stroke stabilized at 0.6 (favoring protected CAS) in 2006.5 Thromboemboli may result from catheterization of the arch, great vessel origins, and endovascular manipulation of the carotid bifurcation lesion itself. Up to 20% to 40% of strokes in the CAPTURE (Carotid Acculink/Accunet Post-Approval Trial to Uncover Unanticipated or Rare Events) registry resulted from access difficulties during cannulation of the aortic arch vessels.6 Access problems appear to be related to operator experience. In EVA3S (Endarterectomy Versus Angioplasty in Patients with Severe Symptomatic Carotid Stenosis), the French trial of CAS versus CEA in purely symptomatic patients, 5% of CAS patients were converted intraoperatively to CEA owing to access problems, with 15% of these patients having a stroke before CEA.7 The trial has been widely criticized for the relative inexperience of those operators performing CAS. An elegant Italian study sought to evaluate risk for CAS stratified by procedural phase.8 Two distinct timelines were identified: 195 CAS procedures were performed between 2001 and 2003, and 432 CAS procedures were performed between 2004 and 2006. Five major procedural steps were considered: Phase 1, aortic arch and great vessel catheterization; Phase 2, placement of the EPD (mostly of the filter type, the Mo.Ma proximal EPD being used in just over 7% of cases); Phase 3, stent/balloon phase, to include EPD retrieval for the majority of devices used (i.e., distal filters); Phase 4, the first 24 hours “off table”; and Phase 5, the late postinterventional period (i.e., 24 hours to 30 days). Four of ten major strokes occurred during Phase 1 (i.e., catheterization of the arch and great vessel origin ipsilateral to the lesion to be treated at the carotid bifurcation). With time (and experience), the major stroke/death rate fell from 3.1% to 0.9%. (P = 0.047) between the early and later timeframes. • Agitated contrast, resulting primarily in gaseous microemboli during the drawing up of contrast and its injection9 • Catheterization of the great vessel origin • Endovascular manipulation of plaque • Plaque prolapse through stent struts • Platelet aggregates on stent struts following stent deployment prior to stent endothelialization Microembolic signals (MES) can be assessed on transcranial Doppler (TCD) of the middle cerebral artery and may be applied either ipsilateral to the lesion being treated or bilaterally. Cellular edema on diffusion-weighted magnetic resonance imaging (DWMRI) of brain, manifesting as new white hyperintense lesions, are a further marker of cerebral insult. Bright lesions that have matched restricted diffusion on apparent diffusion coefficient (ADC) maps can date the cerebral insult as acute (i.e., up to 7-10 days of age). DWI hyperintensities have been used to compare the magnitude of the embolic burden between different carotid interventional strategies. During carotid stenting, MES counts are generally increased in three phases: predilation, stent deployment, and postdilation, with the highest counts during stent deployment or aggressive postdilation.10 Although MES and DWI hyperintensities are useful for research purposes, representing a proxy for neurologic injury that may or may not be subclinical, the fate and clinical relevance of these findings is incompletely understood.11 The International Carotid Stenting Study (ICSS) was a one-to-one randomized comparison of CAS versus CEA in standard-risk patients in a U.K.-based international trial.12 In a substudy of the ICSS trial, 231 patients had preprocedural, 1 to 3 days postprocedural, and 1-month postprocedural DWMRI scans to compare the microembolic penalty following CEA to that of CAS.13 The new white lesion rate was 50% for largely filter-protected CAS and 17% for CEA. There were fewer larger lesions following CEA, and many more smaller lesions for CAS (i.e., the same mean volume of brain was affected by CAS and CEA). Five percent of lesions were in the territory contralateral to the carotid artery being treated in CAS patients, compared with 1% of contralateral lesions following CEA. In 33% of lesions detected on postprocedure DWMRI in CAS patients, the lesions were evident on fluid attenuation inversion recovery (FLAIR) imaging at 30 days, implying some level of permanent injury, compared with 8% for CEA patients. Accepting this, an analysis of cognitive function as a further subset analysis of the ICSS dataset revealed no difference in neuropsychometry between treatment limbs, despite an excess of DWMRI lesions following CAS.14 Two small randomized trials have sought to compare control of the microembolic burden with the Mo.Ma proximal EPD and filter protection. The first (recruiting 53 patients with lipid-rich plaque as assessed on computed tomographic angiography [CTA]) demonstrated significantly more MES with filters than with the Mo.Ma: 101 ± 53 versus 22.5 ± 19 and substantially more DWMRI new lesions with filters. The difference in this endpoint did not reach statistical significance because the study was underpowered for the DWI surrogate (N = 45).15 The latest trial recruited 62 patients and demonstrated an 87.1% new white lesion rate following filter-protected CAS and a 45.2% new white lesion rate following Mo.Ma-protected CAS, and there were significant differences with respect to mean number of lesions and mean volume of brain affected in favor of the Mo.Ma. This differential in favor of proximal protection was maintained regardless of either symptom or octogenarian status.16 Prospective cohort analyses show comparable and consistent discrepancies between proximal and distal filter devices in favor of proximal devices. In a prospective analysis of 21 patients undergoing Mo.Ma-protected CAS and 21 undergoing filter-protected CAS, Schmidt et al. demonstrated a total MES count of 57 ± 41 for the Mo.Ma device and 196 ± 84 for the filter (P < 0.0001). Sheath and/or protection device placement and retrieval were noted to be universally emboligenic stages of the procedure.17 In the DESERVE (Diffusion-Weighted MRI-Based Evaluation of the Effectiveness of Endovascular Clamping During Carotid Artery Stenting with the Mo.Ma Device) study evaluating the Mo.Ma in a multisite E.U. registry of 127 patients, the new white lesion rate was 30%.18 A small but elegant nonrandomized study compared CEA with filter-protected and flow reversal–protected CAS via a femoral route in 42 patients19 (Table e8-1). TABLE e8-1 Comparison of MES for CEA, Filter-Protected CAS, and Flow Reversal–Protected CAS CEA versus filter-protected CAS P = 0.001 CEA versus flow reversal protected CAS P = 0.007 Flow reversal versus filter protection P = 0.05 CAS, Carotid artery stenting; CEA, carotid endarterectomy; MES, microembolic signals. PROOF (Embolic Protection System: First-in-Man Study) was an analysis of the MICHI system (high flow rate reversed flow via a mini-incision in the ipsilateral CCA, thereby avoiding catheterization of the arch and great vessel origins). A subgroup of patients (n = 31) were examined with DWMRI at 24 to 48 hours post procedure, and the scans were read by two independent neuroradiologists in the United States. New ischemic lesions were identified in 16% of patients, comparing favorably to the CEA arm of the ICSS DWI substudy, where 17% had new ischemic lesions.20 This is the first time a new white lesion rate comparable to CEA has been demonstrated by a stenting strategy, and it most likely results from the avoidance of catheter/guidewire manipulation in the aortic arch. The first carotid angioplasty and stenting procedure with embolic protection was performed by Jacques Theron, using a homemade coaxial system that allowed temporary distal internal carotid artery occlusion during stent placement.21 This formed the basis of the subsequent PercuSurge GuardWire (Medtronic). Further examples of distal occlusive devices include the newer in-stent occlusion TwinOne system (Minvasys, Gennevilliers, France) (Fig. e8-1), a variant of Theron’s original concept, and the FiberNet system (initially Lumen Biomedical, now Medtronic). The TwinOne system limits cerebral protection to the poststenting angioplasty phase, one of the most emboligenic phases of the CAS procedure.22 The device has a compliant 0.014-inch wire-mounted balloon that is inflated within the leading cephalad end of the stent. The lesion is predilated up to 2 mm if required, and a stent is placed, both stages being unprotected. The TwinOne occlusion balloon is placed and, with a further angioplasty balloon, preloaded onto the same wire. The TwinOne balloon is inflated, and gentle contrast injection is performed to ensure adequate occlusion by the TwinOne balloon. The stent is balloon dilated with the second balloon. Following dilation, there is gentle aspiration of the standing column via the guiding catheter, and the TwinOne balloon is deflated and removed.
Embolic Protection Devices
Evidence of Improved Clinical Outcomes with Protected Carotid Artery Stenting
Embolic Burden of Carotid Artery Stenting
Macroemboli
Microemboli
Assessing Microemboli
Differential Control of Microembolic Burden of Carotid Artery Stenting
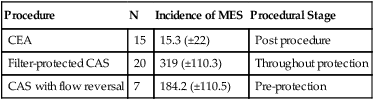
Procedure
N
Incidence of MES
Procedural Stage
CEA
15
15.3 (±22)
Post procedure
Filter-protected CAS
20
319 (±110.3)
Throughout protection
CAS with flow reversal
7
184.2 (±110.5)
Pre-protection
Evolution of Embolic Protection Devices
Distal Devices—Occlusive
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine