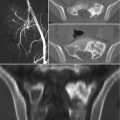
Fig. 25.1
An 11-year-old girl with external popliteal nerve deficit of 10 days duration. (a, b) Axial CT of the sacrum showed an osteolytic lesion involving the sacroiliac joint; biopsy showed aneurysmal bone cyst. (c, d) Digital subtraction angiography shows the high pathological vascularization originating from the pathologic feeding vessels of the tumor; NBCA embolization was done. (e) CT of the sacrum at 2-month follow-up because of persistent pain; a second NBCA embolization was done (f) CT of the sacrum at 3-month follow-up after the second embolization show partial ossification of the tumor. (g) CT at 1-year follow-up show almost complete ossification of the tumor; the patient is asymptomatic
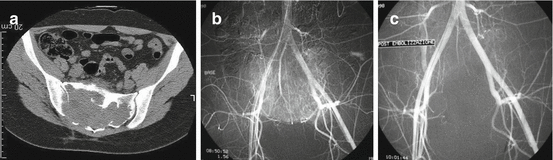
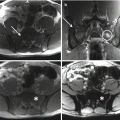
Fig. 25.2
(a) Axial CT of the pelvis of a 30-year-old man with a giant cell tumor of the sacrum. (b) Digital subtraction angiography shows the pathological tumor vascularization. (c) Digital subtraction angiography after NBCA embolization shows complete occlusion of the pathological tumor vessels
Serial embolization is typically performed at 4–6-week intervals until symptomatic improvement occurs, or the tumor’s vascularity disappears. Success of embolization is confirmed clinically by improvement of pain or with imaging using post-procedural angiography, MR imaging, or CT. Lesion ossification after embolization of aneurysmal bone cysts is considered a treatment success. Recurrence of symptoms should be evaluated with MR imaging, CT, or angiography. If there is an increase in tumor size or evidence of increased vascularity, then embolization should be repeated [15]. Puri et al. [22] consider serial embolization for benign sacral tumors that extend above S3, in order to retain bladder and bowel control and minimize neurological dysfunction, in patients with preoperatively intact bladder and bowel function. In these cases, supplementary administration of parenteral bisphosphonates could be proved beneficial [22].
25.3 Embolization for Primary Tumors of the Sacrum
The complexity of the sacral neuroanatomy and its close relationship with vital organs adds additional challenges in the treatment of malignant tumors of the sacrum. Their management is governed by an interplay of complex factors, which includes their pathology, the extent of the disease, and presenting neurological symptoms [22]. Embolization has been used for such tumors as an adjunct to surgery, chemotherapy, and radiation therapy, or as palliative treatment [3]. Some tumors arising from the neural elements of the sacrum such as presacral schwannomas, neurofibromas, sacral meningiomas, malignant peripheral nerve sheath tumors, and intradural spinal tumors often are vascular and therefore responsive to embolization [22, 23]. In these cases, preoperative embolization is a useful adjunct to surgery to reduce blood loss during surgery and facilitate resection [22, 23]. In a recent study [23], the authors embolized preoperatively giant sacral neurogenic tumors, with a mean tumor size of 17.5 cm. All surgical procedures were performed through a single posterior approach, though most of the tumors were located above S3 level. The low recurrence and mortality rates, as well as the good functional outcome, were attributed to the preoperative embolization, which also facilitated surgeons’ access to the tumor mass through a single posterior approach in all cases.
Chordomas are rare, slow-growing primary malignant bone tumors, thought to originate from embryonic remnants of the primitive notochord. They arise at the midline of the axial skeleton, most commonly at the sacrococcygeal (30–50%) region. Wide en bloc resection with tumor-free margins is considered the key to successful treatment. However, the special anatomic characteristics of the sacrococcygeal region, and the tumor’s invasive nature, allow for a marginal or an intralesional tumor excision, rather than a wide one. Reducing the blood loss during the operation is a measure that could help the surgeon remove the tumor en bloc. In a recent study [24], the authors performed transcatheter arterial embolization of the main arteries that supplied the sacral chordomas. They reported significantly low intraoperative blood loss, and good functional outcomes with acceptable recurrence and mortality rates. These authors advocated that preoperative embolization can significantly decrease intraoperative blood loss, make the surgical field clear, possibly eliminate the need for using an anterior approach, and facilitate the maximal removal of the sacral chordoma [24].
25.4 Embolization for Metastases of the Sacrum
The spinal column is the most common site for metastatic bone disease, with an incidence of spinal metastases of about 20 times higher than that of primary spinal tumors [25]. If not all, most of the metastatic bone tumors are hypervascular; some are highly hypervascular due to angiogenetic factors, cytokines, and bone-resorbing factors being produced, which also contribute to bone failure. The hypervascularity of the metastatic tumors does not apply though universally. Tumors of typically high hypervascularity such as renal and thyroid, not rarely, have been found with low vascularization, whereas tumors considered non-hypervascular, such as lung and breast, have shown hypervascularity [26]. These observations emphasize the need of careful patient selection and a treatment strategy plan. Apart from the abovementioned spinal metastatic tumors, embolization has been also reported in metastases from melanomas, pheochromocytomas, and sarcomas, as well as from colon, prostate, uterus, ovarian, hepatobiliary, gastric, pancreatic, parotid, and lacrimal gland cancer and cancer of unknown origin [25, 26]. In general, embolization can serve as a palliative, as well as an adjuvant preoperative treatment in metastatic disease of the sacrum [20, 25]. It may successfully reduce intraoperative blood loss, improving surgeons’ visibility and accessibility to the tumor, therefore, facilitating curettage, and reduce intraoperative morbidity and complications (Fig. 25.3). Preoperative embolization can also reduce the size, arrest tumor growth, alleviate pain, and shorten hospital stay [15].
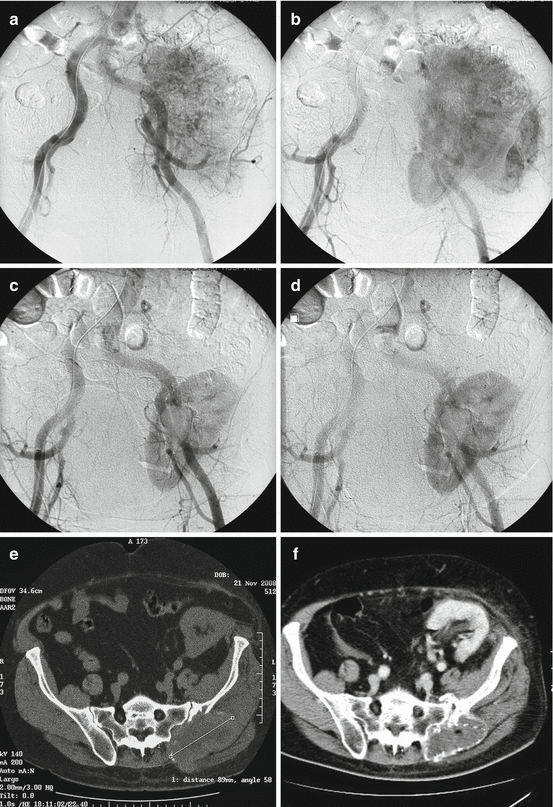

Fig. 25.3
A 63-year-old man with a painful sacroiliac metastasis from renal cancer originating at a transplanted kidney. (a, b) Digital subtraction angiography shows extensive tumor vascularization. (c, d) Digital subtraction angiography after NBCA embolization shows occlusion of the tumor vessels. (e) CT of the pelvis and sacrum at the time of embolization. (f) CT of the pelvis and sacrum at the 12-month follow-up show complete ossification of the metastatic lesion; the patient is asymptomatic
There is lack of evidence in literature, however, regarding the outcomes of preoperative embolization in metastatic tumors of the sacrum [14, 15, 20, 25]. This could be attributed to the fact that surgical resection of a sacral metastasis consists a great risk accompanied with major complications and doubtful clinical benefits for the patient. Palliative embolization, on the other hand, has been reported as a satisfactory therapeutic alternative for these patients [14, 15, 20, 25]. Recently, Facchini et al. [25] reported good results in a large patient series with spinal metastases treated with N-2-Butyl-cyanoacrylate (NBCA) embolization. A fifth of patients had metastases of the sacrum, and good or moderate clinical response was noticed in 97% of the patients. Interestingly though, five patients, who showed no response, were patients with metastases of the sacrum. The mean duration of pain relief was 9.2 months, and for the patients who experienced recurrent intense pain similar to pre-embolization pain, the embolization was repeated one or two more times with significant pain relief and reduction in analgesics at the last follow-up. The authors concluded that embolization with NBCA should be considered for pain palliation of patients with metastases of the spine and sacrum, as it is a safe and effective procedure.
25.5 Palliative Embolization
The exact mechanism of pain relief following embolization is still unclear. In 1979, Chuang et al. [27] suggested that especially in hypervascular neoplasms the vascular occlusion decreases the size of the tumor and slows its progression. Subsequently, there is a decrease in the pressure of expansion or in the stretching of the periosteum, which contains the nerve fibers that are responsible for pain. This theory remains the only viable explanation of the pain relief mechanism of embolization [14]. The palliative properties of this technique can be extended in primary sacral sarcomas as well. In a recent study [28], selective embolization has been used for palliation of pain in patients with locally advanced sarcomas. In this cohort, patients with primary sacral sarcomas, recurrent or unresectable, responded satisfactory to this treatment with almost complete or moderate pain relief, along with a minimum 50% reduction of daily analgesic doses. Therefore, embolization should be recommended as a safe and effective local palliative treatment, providing optimum pain relief and improving the quality of the remaining life of these patients, by offering the least discomfort with the minimum possible complications [28].
In the question, which factors should be taken into consideration as predictors of the success of an embolization, the answer is the tumor’s vascular properties before and after embolization (the tumor’s vascularity, the completeness of the occlusion, and the availability of collateral circulation) along with the use of the appropriate embolic agent [25, 27, 28]. Therefore, selective and superselective embolization of the pathological tumor feeding vessels may provide for successful embolization.
Pain relief after embolization may be temporary; however, it occurs rapidly. Indeed, the pain-free period may averagely last 8–9 months; however the patients are relieved immediately, as soon as 12 h up to several days after the embolization procedure, usually within the first week from the procedure; this rapid response, of course, outmatches other methods, such as radiotherapy and chemotherapy [14, 25, 28, 29]. Tumors’ size should not be considered when planning an embolization procedure, since it does not seem to affect the outcomes [25]. Therefore, embolization can be proved superior to thermal ablation and cryoablation as well, because sacral tumors are usually large, and ablations should be performed only in painful lesions of <3 cm of maximum diameter. Sacral tumors are also very close to neurovascular bundles; therefore, they cannot be adequately ablated, due to the risk of injuring those fine tissues [28].
25.6 Complications
Common embolization-related complications in most patients include nausea, emesis, low-grade fever, and pain, which usually last 3–7 days; this is known as post-embolization syndrome that has been reported in 18–86% of cases [14, 15]. The rates of major post-embolization complications are low ranging from 1 to 2%, or less [14, 29]. Ischemic neuropathy can result in motor and sensory deficits in the pelvis and lower extremities, and it is a potential complication of any pelvic embolization. During pelvic embolizations through the iliac artery and its major branches, ischemic neuropathies of the sciatic and femoral nerves may occur if neural vessels were occluded. To prevent these complications, the posterior branch of the internal iliac artery and the inferior gluteal artery must be spared at embolization. Complications related to the embolic agents have also been reported. Additionally, the neuraxis or the sacral plexus of nerves can be injured. Therefore, care must be taken to identify and avoid embolization of the neurovascular anatomy. Rectal ischemia can result from superior hemorrhoidal artery embolization. Any embolization of sacral tumors may result in injury to nontargeted tissue including muscle infarction, injury to the skin, or injury to the colon or other organs [14, 15].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree