Endocrine Imaging
William H. Martin
Martin P. Sandler
M. Reza Habibian
 |
This chapter includes a series of scintigraphic case presentations with correlative imaging of the thyroid, parathyroid, and adrenal glands. Imaging of neuroendocrine tumors is addressed in Chapter 8.
Thyroid Gland
The shield-shaped thyroid gland is normally positioned anterior and lateral to the cricoid cartilage, although it may be located more superiorly anterior to the thyroid cartilage or more inferiorly anterior to the trachea. The thyroid contains two lobes, each with superior and inferior poles, an isthmus, and often a pyramidal lobe that originates from the isthmus or the medial aspect of either lobe. The pyramidal lobe develops along the distal thyroglossal duct, thus its location. Similarly, thyroid tissue may be ectopically located anywhere along its embryonic migration track—i.e., as a lingual thyroid at the base of the tongue, within a vestigial midline thyroglossal duct cyst, or further caudally within the mediastinum. The normal thyroid weighs 15 to 20 g; a mildly enlarged 30-g gland is barely palpable, whereas a 40-g gland is easily palpable and often visible with deglutition if the neck is extended. Goiters as large as 60 g or more are easily visible in the unextended neck.
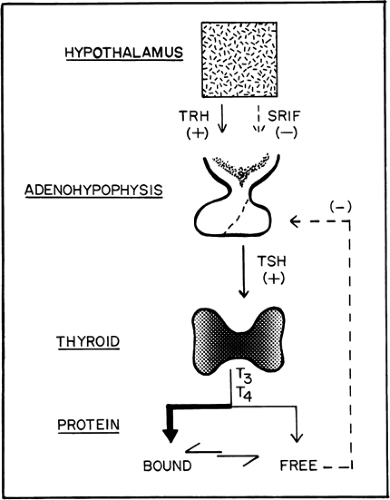
Within the regulation of the hypothalamic–pituitary axis, the functions of the thyroid gland include the trapping of iodine, the synthesis and storage of hormones, and the release of these hormones into the circulation (Fig. 1.A). This ability to trap iodine is not unique to the thyroid gland, because trapping also occurs in the salivary glands, gastric mucosa, and breast, but none of these other tissues is able to organify the trapped iodine to synthesize thyroid hormones.
Thyroid Gland Scintigraphy
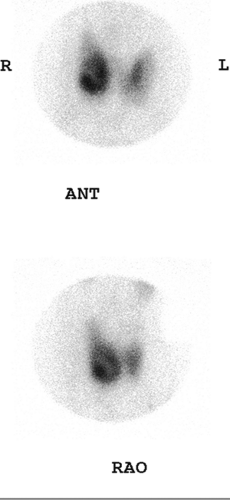
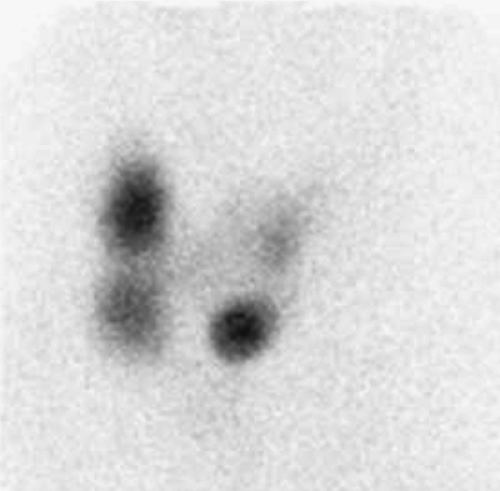
Technetium 99m- (99mTc) pertechnetate or iodine 123 (123I) may be used to image the thyroid gland. The advantage of using 123I as compared with 99mTc is that 123I is not only trapped, as is pertechnetate, but is also organified, thus providing a more accurate representation of thyroid function. Owing to its low cost and ready availability, 99mTc-pertechnetate is more frequently used and, in most instances, provides comparable diagnostic information. To obtain the maximum image quality and spatial resolution, a pinhole collimator is used to acquire anterior and oblique images. Oblique imaging may sometimes demonstrate a small posterior nodule (Fig. 1.B, lower) not seen on the anterior images (Fig. 1.B, upper).
131I is used primarily for the determination of thyroid uptake. Although 131I is used routinely to detect recurrent and metastatic thyroid cancer, the high radiation dose from its
beta emissions as well as its high-energy gamma emission (364 keV) precludes its use in routine thyroid imaging.
beta emissions as well as its high-energy gamma emission (364 keV) precludes its use in routine thyroid imaging.
Thyroid hormone therapy with either thyroxine (T4, Synthroid®) or tri-iodothyronine (T3, Cytomel®) will suppress the hypothalamic–pituitary–thyroid axis and markedly diminish the uptake of radionuclides used for thyroid imaging. Flooding the vascular pool with iodine by administering exogenous iodine, as with iodinated contrast agents, saturates the thyroid with iodine and similarly suppresses thyroidal uptake of radioiodine.
Parathyroid Glands
The parathyroid glands are usually situated along the posterior aspect of the lateral thyroid lobes. They normally measure about 6 × 3 mm in width and weigh approximately 35 mg each. There are usually four parathyroid glands, two upper and two lower. Supernumerary glands have been reported in 2% to 6% of adults and may occur anywhere from the level of the submandibular glands to the arch of the aorta.
Parathyroid Gland Scintigraphy
Although technetium 99m/thallium 201 (99mTc/201Tl) subtraction imaging has been used to effectively identify parathyroid pathology, 99mTc-sestamibi (99mTc-MIBI), a lipophilic cation used for both myocardial and tumor imaging, is now used. Distribution of 99mTc-MIBI is proportional to blood flow, and once intracellular, it is sequestrated primarily within the mitochondria in response to the electrical potential generated across the membrane bilayers of both the cell and mitochondria. The large number of mitochondria present in the cells of parathyroid adenomas may be responsible for the avid uptake and slow release of 99mTc-MIBI in parathyroid adenomas compared with surrounding thyroid tissue. Detection and localization of parathyroid adenomas with 99mTc-MIBI can be performed by either a single radiopharmaceutical injection or a dual-isotope procedure using a low-energy high-resolution collimator with immediate and 2- to 3-hour delayed anterior (and sometimes oblique) imaging.
Adrenal Glands
Each adrenal gland lies in the retroperitoneal perinephric space, near the upper poles of the kidneys. Although CT and MRI are used as the primary imaging modalities for adrenal pathology, scintigraphic techniques are important in several specific instances.
Adrenal Medulla Scintigraphy
The adrenal medulla produces and secretes the principal catecholamine epinephrine. Metaiodobenzylguanidine (MIBG) is a guanethidine analog that localizes via the norepinephrine reuptake mechanism into catecholamine storage vesicles of adrenergic nerve endings and cells of the adrenal medulla. MIBG localizes in other organs with rich adrenergic innervation, including the heart, spleen, and salivary glands. Both 131I-MIBG and 123I-MIBG are commercially available for imaging of neuroendocrine tumors, but 123I-MIBG is not FDA-approved in the United States. 123I-MIBG has more favorable dosimetry and imaging characteristics, permitting the acquisition of single photon emission computed tomography (SPECT) and SPECT-CT images.
With 123I-MIBG, activity within normal adrenals as well as the uterus and prostate can be seen. Owing to radiolysis, activity within the bowel and bladder is physiologic.
Patients are pretreated with inorganic iodine (Lugol’s solution or potassium iodide), 1 to 2 mg/kg per day, beginning 1 to 2 days prior and continuing for 1 week following the administration of 131I-MIBG and for 3 days after 123I-MIBG injection. After administration of 131I-MIBG, imaging is delayed to 48 and 72 hours, whereas imaging with 123I-MIBG is performed at 24 hours.
Case 1.1
History: A 43-year-old female was noted to have a palpable nodule in the right lobe of her thyroid. Her thyroid function tests were entirely normal, and she was referred for thyroid scintigraphy (Fig. 1.1 A).
Findings: A scintigram of the neck following IV injection 10 mCi 99mTc-pertechnetate showed an area of markedly reduced uptake in the lower pole of the right lobe, corresponding to the palpable mass and representing a hypofunctioning nodule.
Discussion: Some 85% to 90% of thyroid nodules are hypofunctioning, but only 10% of cold nodules are malignant. The remaining hypofunctioning nodules represent degenerative nodules, nodular hemorrhage, cysts, focal thyroiditis, infiltrative disorders such as amyloid, and nonthyroid neoplasms.
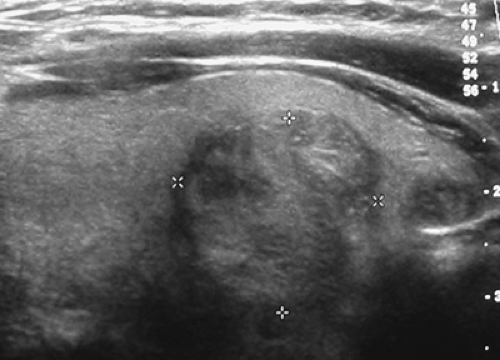
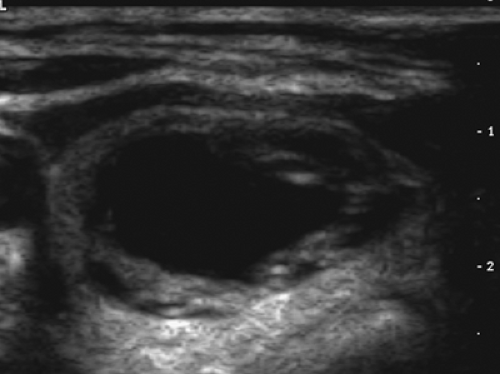
A subsequent ultrasound (Fig. 1.1 B) demonstrated a 1.9-cm solid mass in the lower pole of the right lobe of the thyroid without sonographic findings suspicious of malignancy. A fine-needle-aspiration (FNA) biopsy was benign; annual sonographic surveillance was planned.
Palpable thyroid nodules occur in 5% of women and 1% of men and are detectable in 40% of people by ultrasound, more often in women and in the elderly. Approximately 5% to 10% of these nodules are malignant, depending on age, gender, family history, and prior radiation exposure. In the United States, approximately 24,000 cases of differentiated thyroid cancer are diagnosed annually. The incidence is rising, probably related to increased detectability using ultrasound. Imaging is used in an effort to aid in the differentiation of malignant from benign lesions.
Current guidelines recommend a history and physical exam followed by a serum TSH determination and an ultrasound procedure. Generally the presence of a firm nodule, rapid growth, fixation to the adjacent structures, vocal cord paralysis, ipsilateral cervical adenopathy, male gender, age younger than 20 years or older than 70 years, history of head and neck radiation, and family history of thyroid cancer are significant risk factors suggestive of increased malignant potential.
If the serum TSH is suppressed, a 99mTc-pertechnetate scan is recommended to exclude the presence of a functioning nodule, for which biopsy and ultrasound are unnecessary (see discussion of Case 1.2). If the TSH is normal or elevated, an ultrasound is recommended to document number, size, location, and specific sonographic characteristics of the nodule(s). FNA biopsy is the most accurate and cost-effective tool for evaluating thyroid nodules. Nonpalpable nodules have the same risk of malignancy as palpable nodules of similar size, so nodules identified incidentally on ultrasound or CT require evaluation similar to clinically evident nodules. Figure 1.1 C and Figure 1.1 D demonstrate an incidental hypofunctioning nodule within the medial aspect of the left lobe and isthmus. Nodules smaller than 10 mm need not be biopsied unless suspicious ultrasound characteristics are found or there is a family history of thyroid cancer or a history of radiation exposure of the head or neck. Otherwise, nodules larger than 10 mm are biopsied by FNA using ultrasound guidance, even if they are partially necrotic. Recommendations regarding multinodular goiter are reviewed in Case 1.3. FNA biopsy produces false
negatives in 1% to 3% of cases, so annual ultrasound surveillance is recommended; nodule growth of >20% in diameter is an indication for repeat biopsy.
negatives in 1% to 3% of cases, so annual ultrasound surveillance is recommended; nodule growth of >20% in diameter is an indication for repeat biopsy.
None of the ultrasound findings can be used to either diagnose or exclude malignancy with a high degree of accuracy, but the combination of a solid hypoechoic mass with an irregular border, microcalcifications, and/or increased intranodular vascularity increase the potential for malignancy, mandating biopsy.
Diagnosis: Benign follicular neoplasm.
Case 1.2
History: A 32-year-old female is referred for scintigraphy and 131I uptake (radioactive iodine uptake, or RAIU) owing to the finding of a left neck mass and a suppressed serum TSH value.
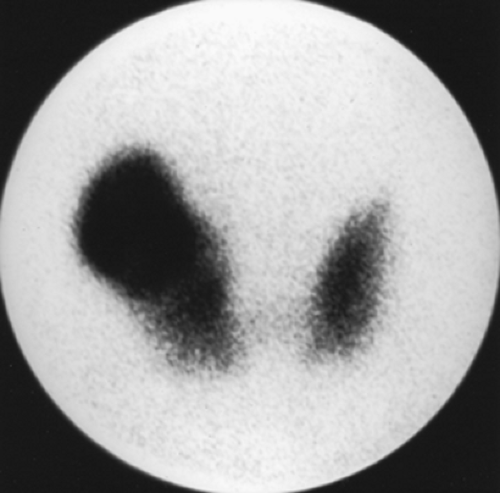
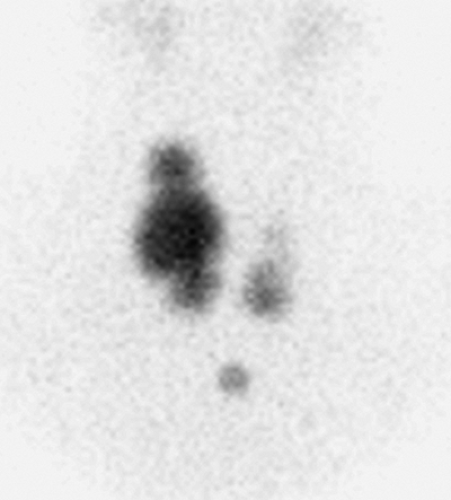
Findings: On the 99mTc-pertechnetate scan (Fig. 1.2 A) there is intense uptake in the palpable left thyroid nodule with complete suppression of all extranodular activity. The RAIU is elevated to 38% at 24 hours.
Discussion: These findings are consistent with an autonomously functioning follicular adenoma. The suppression of all extranodular activity is seen in patients with overt hyperthyroidism. In the context of hyperthyroidism, the solitary hyperfunctioning nodule is virtually always benign; no additional imaging or biopsy is required. Definitive treatment with 131I or surgical resection is indicated to relieve the patient of her hyperthyroidism.
According to the recent American Thyroid Association guidelines for patients with thyroid nodules, scintigraphy for a nodule or multinodular goiter (MNG) is indicated only (1) in patients who have a serum TSH below the lower limit of the normal range, (2) if ectopic thyroid tissue or a retrosternal nodule is suspected, or (3) if an indeterminate FNA biopsy result is suggestive of a follicular neoplasm. If an autonomously hyperfunctioning nodule is not seen on scintigraphy in the patient with an indeterminate biopsy, thyroidectomy is recommended. A recent report of 42 consecutive nodule patients with indeterminate cytologic results found that fluorodeoxyglucose positron emission tomography (FDG-PET) had a sensitivity of 100% and a specificity of 39%, concluding that the use of FDG-PET would reduce unnecessary thyroidectomies by 39% in patients with benign disease. Other authors have reported a high negative predictive value and high sensitivity in the preoperative evaluation of thyroid nodules. Similarly, 201Tl and 99mTc sestamibi are useful adjuncts to FNA cytology in the evaluation of solitary thyroid nodules when the latter is inconclusive (see discussion of Case 1.8).
In a prospective report of 78 patients with solitary hypofunctioning thyroid nodules undergoing thallium scintigraphy preoperatively, 86% of the 65 benign lesions showed less than or equal uptake as compared with normal thyroid tissue at 3 hours, and 85% of the malignant lesions were “hot” on delayed imaging; however, 14% of the benign lesions also showed increased activity on the delayed images. In a report of 71 patients undergoing preoperative 99mTc-MIBI scintigraphy, 91% of the 23 carcinomas were positive with 99mTc-MIBI, and only 11% of the benign lesions were positive. Therefore both 99mTc-MIBI and 201Tl imaging are useful adjuncts to FNA cytology in the evaluation of solitary thyroid nodules, especially when the latter is inconclusive.
Although a functioning thyroid nodule in the euthyroid patient may represent hyperplastic (sensitive to TSH stimulation) tissue, most are autonomously functioning adenomas (AFTNs) arising independently of TSH stimulation. Biochemical hyperthyroidism, often subclinical, is present in 74% of patients at presentation, although overt hyperthyroidism is less common. Over a period of 3 years after detection, 33% of AFTNs enlarge in patients not receiving definitive therapy, and 24% of euthyroid patients develop hyperthyroidism. If overt hyperthyroidism exists, the surrounding normal thyroid tissue will be suppressed, and the TSH level will be undetectable (Fig. 1.2 A).
In euthyroid patients, surrounding extranodular thyroid tissue will be visible (Fig. 1.4 B) and thyroid function studies will be normal; these patients can be followed on an annual basis. Spontaneous cystic degeneration occurs in 27%, manifested by central photopenia; there is little concern for malignancy (Figs. 1.2 C, D). Radioiodine therapy with a dose of 10 to 20 mCi is a simple, safe, and cost-effective mode of therapy, but late hypothyroidism may develop in as many as 25% of such patients. Surgery may be advisable for larger AFTNs, especially if compressive symptoms exist. As an alternative to surgery or 131I treatment, percutaneous ethanol injection (PEI) is successful in alleviating hyperthyroidism in approximately 67% of patients with toxic AFTNs, but this usually requires repeated injections under sonographic guidance; current guidelines recommend PEI only for small AFTNs (volume <5 mL) not yet completely suppressing the surrounding thyroid parenchyma, and then only if such patients are concerned about the occurrence of late hypothyroidism.
In euthyroid patients, surrounding extranodular thyroid tissue will be visible (Fig. 1.4 B) and thyroid function studies will be normal; these patients can be followed on an annual basis. Spontaneous cystic degeneration occurs in 27%, manifested by central photopenia; there is little concern for malignancy (Figs. 1.2 C, D). Radioiodine therapy with a dose of 10 to 20 mCi is a simple, safe, and cost-effective mode of therapy, but late hypothyroidism may develop in as many as 25% of such patients. Surgery may be advisable for larger AFTNs, especially if compressive symptoms exist. As an alternative to surgery or 131I treatment, percutaneous ethanol injection (PEI) is successful in alleviating hyperthyroidism in approximately 67% of patients with toxic AFTNs, but this usually requires repeated injections under sonographic guidance; current guidelines recommend PEI only for small AFTNs (volume <5 mL) not yet completely suppressing the surrounding thyroid parenchyma, and then only if such patients are concerned about the occurrence of late hypothyroidism.
In the evaluation of a nodule appearing hyperfunctioning on 99mTc imaging, one should be aware of the occasional existence of a discordant nodule. Discordant thyroid imaging is dissociation between trapping and organification, measured respectively with 99mTc-pertechnetate and 123I. It occurs in only 2% to 8% of thyroid nodules and is not specific for
malignant disease. A nodule that traps 99mTc (hot) (Fig. 1.2 E) but is unable to organify iodine (cold) (Fig. 1.4 F) is much more likely to be benign than malignant. This ability to trap small anions accompanied by a loss of the ability to organify iodine has been observed in adenomatous goiters, focal thyroiditis, and follicular adenomas, most often seen in multinodular goiters. If it is assumed that 8% of hot nodules with 99mTc are cold with 123I and 10% of those are malignant, then less than 1% of hot nodules seen with 99mTc imaging are malignant. Additional radioiodine imaging of hot nodules identified on a 99mTc scan should probably be reserved for patients deemed at higher risk for malignancy, and initial scintigraphy with 123I rather than 99mTc-pertechnetate should be reserved for patients deemed at high risk for carcinoma, such as children, males, and patients with a prior history of radiation exposure or a family history of thyroid cancer.
malignant disease. A nodule that traps 99mTc (hot) (Fig. 1.2 E) but is unable to organify iodine (cold) (Fig. 1.4 F) is much more likely to be benign than malignant. This ability to trap small anions accompanied by a loss of the ability to organify iodine has been observed in adenomatous goiters, focal thyroiditis, and follicular adenomas, most often seen in multinodular goiters. If it is assumed that 8% of hot nodules with 99mTc are cold with 123I and 10% of those are malignant, then less than 1% of hot nodules seen with 99mTc imaging are malignant. Additional radioiodine imaging of hot nodules identified on a 99mTc scan should probably be reserved for patients deemed at higher risk for malignancy, and initial scintigraphy with 123I rather than 99mTc-pertechnetate should be reserved for patients deemed at high risk for carcinoma, such as children, males, and patients with a prior history of radiation exposure or a family history of thyroid cancer.
Diagnosis: Toxic autonomously functioning follicular adenoma.
Case 1.3
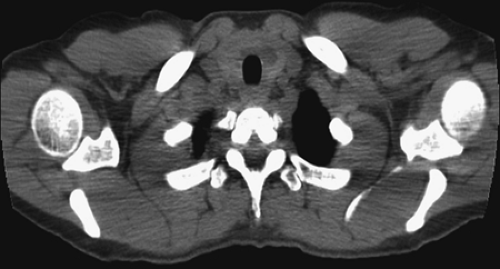
History: A 60-year-old woman with a history of endometrial sarcoma was referred for 99mTc-pertechnetate scintigraphy (Fig. 1.3 A) when a goiter was noted incidentally on CT imaging.
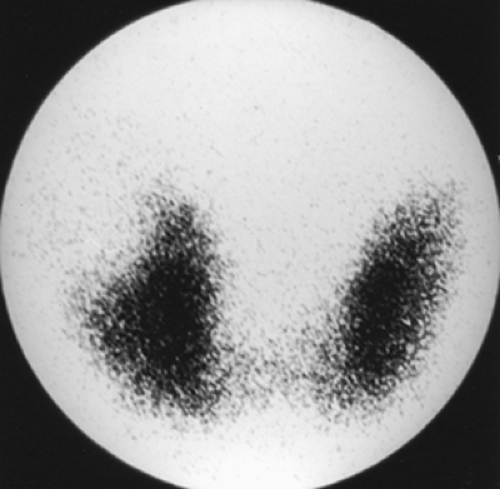
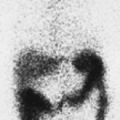
Findings: There is heterogeneous uptake throughout the gland with two hyperfunctioning nodules within the right lobe and a large hypofunctioning nodule within the lower pole of the left lobe. A cobalt 57 (57Co) marker (Fig. 1.3 B) has been placed on a palpable nodule on the left and found to be congruent with the dominant left-lower-lobe cold nodule.
Discussion: The findings are most consistent with a typical MNG, but the left-lower-pole hypofunctioning nodule must be considered worrisome for neoplasm. In view of the history of endometrial sarcoma, the patient underwent thyroidectomy. Histopathology was consistent with MNG with no evidence of thyroid carcinoma or metastatic sarcoma.
Current guidelines affirm that patients with multiple thyroid nodules have the same risk of malignancy as those with solitary nodules, approximately 5% to 10%, depending on age, gender, radiation exposure history, family history, and other factors. If the patient has a low or low-normal serum TSH concentration, a radioiodine scan is recommended to determine the functionality of each nodule larger than 1 to 1.5 cm in diameter. FNA biopsy is recommended only for those isofunctioning or nonfunctioning nodules that demonstrate suspicious sonographic features, such as hypoechogenicity, irregular borders, microcalcifications, and/or intranodular vascularity. If none of the nodules has a suspicious sonographic appearance and multiple sonographically similar coalescent nodules are present, the likelihood of malignancy is low enough that only the largest nodule should be biopsied. If nodular growth of more than 20% in diameter and more than 2 mm occurs at serial follow-up ultrasound, repeat ultrasound-guided biopsy is recommended owing to the 1% to 3% rate of false-negative cytology results at initial FNA.
The development of MNG is related to cycling periods of stimulation followed by involution; it may be idiopathic or occur as a result of endemic iodine deficiency. Over time, the gland enlarges and evolves into an admixture of fibrosis, functional nodules, and nonfunctioning involuted nodules. Scintigraphically, the MNG is a heterogeneously appearing, asymmetrically enlarged gland with multiple cold, warm, and hot areas of various sizes (Figure 1.3 A). The differential diagnosis includes autoimmune Hashimoto’s thyroiditis, multiple adenomata, and multifocal carcinoma.
In the patients with hyperthyroidism, the 24-hour RAIU in toxic nodular goiter may be elevated, but more frequently it is within the high-normal range. Treatment of toxic MNG can be accomplished with 131I administration or by thyroidectomy with similarly good outcomes. Nontoxic MNG requires treatment if compressive symptoms are bothersome. In the patient with comorbidities, 131I therapy, 30 mCi, often with rhTSH stimulation, can effect a 40% decrease in gland volume over the first year and 60% by the end of the second year with alleviation of compressive symptomatology.
Diagnosis: Multinodular goiter with benign dominant cold nodule.
Case 1.4
History: A 24-year-old female patient presented with a history of recent weight loss, palpitations, anxiety, and heat intolerance. The clinical exam revealed tremulousness, moist palms, tachycardia, and a 60-g nontender goiter with no apparent nodularity by palpation. An 131I uptake (RAIU) and 99mTc thyroid scan were performed (Fig. 1.4 A). Four-hour and 24-hour RAIU values were 46% (normal = 10% to 15%) and 62% (normal = 15% to 25%), respectively.
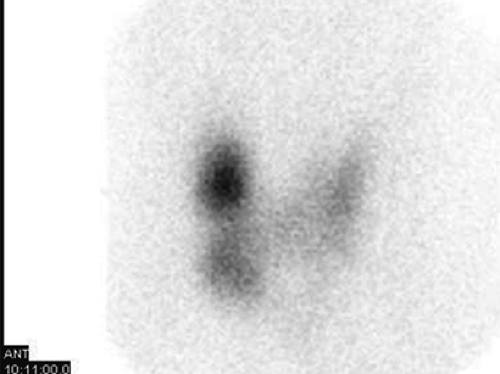
Findings: There is homogenous uptake of 99mTc by the thyroid gland (250,000 counts in 60 sec) with a convex contour to the gland (Fig. 1.4 A). No nodularity is present. Mild linear activity originates from the medial aspect of the left thyroid lobe. Note the relative absence of background and salivary gland activity secondary to increased thyroidal uptake. A 57Co marker has been placed on the suprasternal notch.
Discussion: The scintigraphic finding of diffuse toxic goiter and an elevated RAIU in a young woman with hyperthyroidism is diagnostic of Graves’ disease. The pyramidal lobe, a remnant of the distal thyroglossal duct, is identified in less than 10% of euthyroid patients but is visualized in as many as 43% of patients with Graves’ disease (Fig. 1.4 A). The hyperthyroidism of Graves’ disease is often accompanied by exophthalmos and sometimes pretibial myxedema.
Hyperthyroidism is a clinical syndrome that results from supraphysiologic levels of thyroid hormones and may occur as a consequence of numerous disease processes (Table 1.1). Clinical history and physical examination combined with serum hormone and antithyroid autoantibody levels, thyroid scintigraphy, and RAIU measurement usually allow identification and differentiation of the various etiologies.
TABLE 1.1 Classification of hyperthyroidism | ||
|---|---|---|
|
The presence of thyroid stimulatory antibodies (TSIg) in patients with Graves’ disease results in both increased trapping and organification by the thyroid gland. Scintigraphic imaging of patients with Graves’ disease using 99mTc or 123I reveals diffusely increased thyroidal activity with minimal background and salivary gland activity (Fig. 1.4 A). The gland size will frequently but not always appear enlarged, sometimes asymmetrically. It occurs primarily in young women but also in children and in the elderly.
Patients with diffuse toxic goiter will, in most cases, have an increased RAIU at 4 and 24 hours. Occasionally, patients with Graves’ disease may have a normal 24-hour RAIU but an elevated 4-hour RAIU due to rapid 131I turnover (4hr/24hr RAIU >1). The low RAIU (usually ≤5%) of hyperthyroid patients with subacute or autoimmune thyroiditis is easily differentiated from the high-normal (20% to 30%) uptake seen in some patients with Graves’ disease or those with toxic nodular goiters.
The thyroid scan aids in the differentiation of toxic nodular goiter from Graves’ disease. In a series of 178 patients with hyperthyroidism and no suspicion of thyroid nodularity, 152 patients (85%) had typical Graves’ disease but 9% had
functioning or nonfunctioning nodules, 3% had unexpected subacute thyroiditis, and 3% had AFTNs. Although a cold nodule in a diffuse toxic goiter may represent a functioning but TSH-dependent adenoma (Marine-Lenhart syndrome), malignancy should be excluded by biopsy or thyroidectomy. Therefore scintigraphy is important in the management of patients with Graves’ disease, since 15% of those patients may have associated nodularity or another diagnosis such as AFTN or subacute thyroiditis.
functioning or nonfunctioning nodules, 3% had unexpected subacute thyroiditis, and 3% had AFTNs. Although a cold nodule in a diffuse toxic goiter may represent a functioning but TSH-dependent adenoma (Marine-Lenhart syndrome), malignancy should be excluded by biopsy or thyroidectomy. Therefore scintigraphy is important in the management of patients with Graves’ disease, since 15% of those patients may have associated nodularity or another diagnosis such as AFTN or subacute thyroiditis.
Figure 1.4 B is the scan of a 66-year-old female presenting with a history of fatigue, weakness, palpitations, and dyspnea on exertion. Clinical exam revealed an irregularly, irregular rhythm, bibasilar rales, and a 40-g nodular, firm goiter. Her 24-hour RAIU was 23%. Note the heterogeneous activity throughout the thyroid gland, with several large nodules demonstrating intense trapping. A 57Co marker is at the suprasternal notch. In the context of hyperthyroidism, these findings would be consistent with toxic MNG, probably complicated by atrial fibrillation and mild high-output congestive heart failure. Toxic nodular goiter may be due to multiple nodules (Fig. 1.4 B) or a solitary hyperfunctioning nodule (Fig. 1.4 C).
Patients with toxic nodular goiter are typically between the fourth and fifth decades of life and are more likely to have cardiac complications. On the other hand, patients with
solitary toxic nodules are often younger, varying in age from adolescence to adulthood. Uninodular autonomously functioning adenomas are monoclonal neoplasms, but the pathogenesis of toxic MNG is less certain (see discussion of Case 1.3).
solitary toxic nodules are often younger, varying in age from adolescence to adulthood. Uninodular autonomously functioning adenomas are monoclonal neoplasms, but the pathogenesis of toxic MNG is less certain (see discussion of Case 1.3).
Scintigraphically, toxic MNG (Fig. 1.4 B) appears as focal areas of increased and decreased radionuclide activity scattered throughout the gland. The areas of increased activity represent islands of autonomously hyperfunctioning tissue, whereas the areas of decreased activity are areas suppressed by excessive thyroid hormone levels. Background and salivary activity are often more prominent than that seen in patients with Graves’ disease, and the RAIU is often within the high-normal range rather than elevated.
Hyperthyroidism due to Graves’ disease or toxic nodular goiter may be treated with 131I administration. Antithyroid drug therapy achieves a permanent remission in only 10% to 14% of Graves’ patients and is not a logical long-term option in toxic nodular goiter. Although thyroidectomy is effective and complications are infrequent, surgery is performed only occasionally at present, usually in patients who are unable to accept alternative therapies or who have extremely large goiters with compressive symptoms. Radioiodine therapy is effective, practical, inexpensive, and available on an outpatient basis.
131I should not be administered in the absence of an elevated or high-normal RAIU and confirmation of biomedical hyperthyroidism. An elevated RAIU aids in excluding other etiologies of hyperthyroidism, such as thyroiditis, iodine-induced hyperthyroidism, and factitious hyperthyroidism, all of which are associated with a low RAIU. Rarely, hyperthyroidism with diffuse goiter and elevated RAIU may be caused by excessive secretion of human chorionic gonadotropin by a trophoblastic tumor or by inappropriate secretion of TSH by a functioning pituitary adenoma.
The patient must be counseled prior to therapy regarding the advantages and disadvantages of alternative therapies. Antithyroid drug therapy requires frequent outpatient visits for monitoring and dosage adjustment and may rarely be associated with life-threatening agranulocytosis. Minor toxicity, such as skin rash, fever, hepatitis, and arthalgias, occurs in up to 5% of patients. In addition to the morbidity and rare mortality associated with thyroidectomy, there is a 25% to 50% incidence of postsurgical hypothyroidism as well as a 5% to 20% relapse rate. Because iodide readily crosses the placenta, a pregnancy test is mandatory prior to administration of 131I therapy. Exposure of the fetus to 131I after the 10th week of gestation may result in severe fetal hypothyroidism.
The effectiveness of radioiodine treatment for hyperthyroidism is due to radiation-induced cellular damage resulting from high-energy beta emissions, the magnitude of which is directly proportional to the radiation dose received by the thyroid gland. Some practitioners have adopted a fixed-dose administration of 10 to 15 mCi for all patients. Other physicians calculate a dose of 120 to 150 μCi/g of thyroid tissue for the usual patient with Graves’ disease. Even higher dosages of 150 to 200 μCi/g may be used to produce a more rapid response in patients with severe hyperthyroidism or in typically more radioresistant cohorts such as children/adolescents, recent exposure to propylthiouracil (PTU), prior failed therapy, and those with high iodine turnover. Although estimation of thyroid size by palpation is relatively accurate for glands weighing 60 g or less, the degree of inaccuracy increases in larger glands. Ultrasound can provide a more accurate estimation of size. The calculation is made as follows: administered μCi = μCi/g desired × gland weight (g) × 100 ÷ RAIU (24 hours).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree