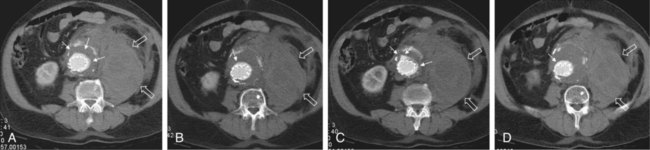
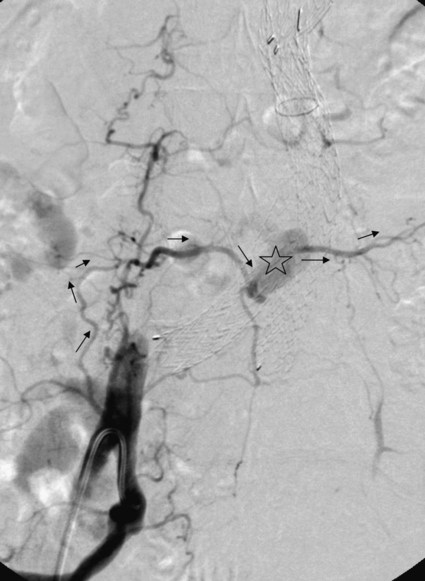
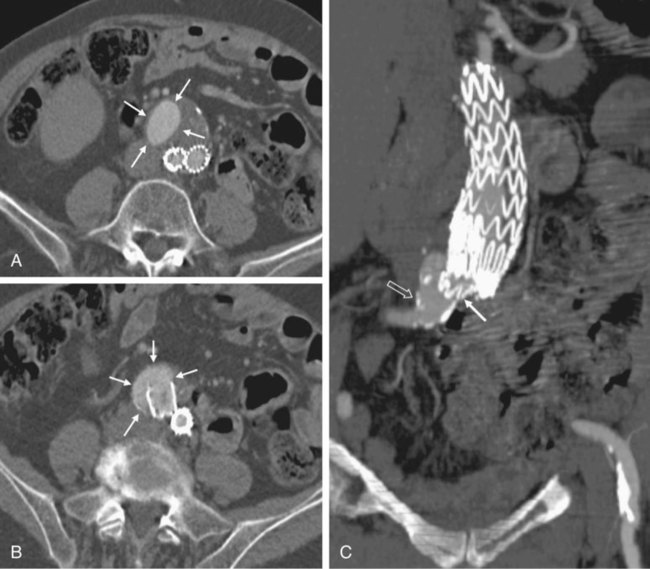
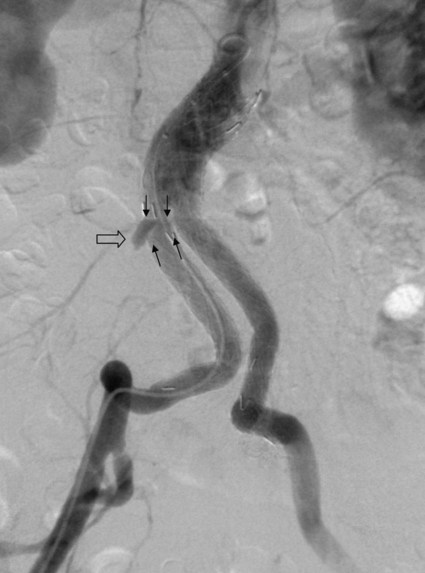
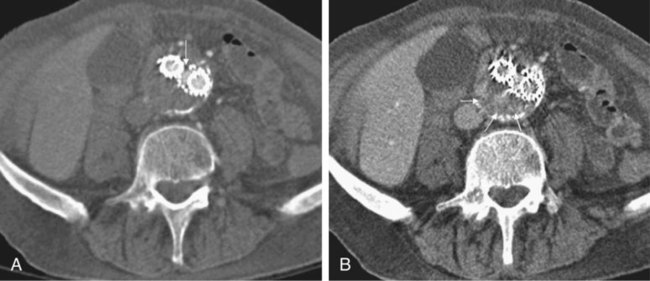
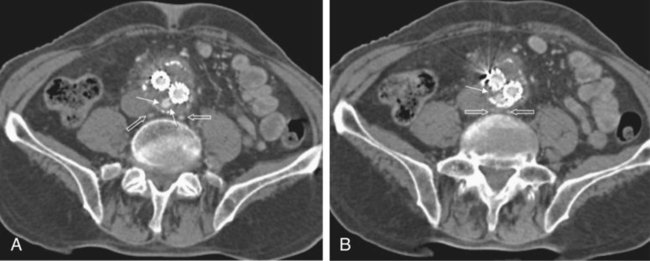
Endovascular aneurysm repair (EVAR) of infrarenal abdominal aortic aneurysms (AAA) has become a widespread procedure of late. Two recently published prospective trials have shown that EVAR of AAAs produces clinical results similar to those of open surgery after observation periods of 6 and 10 years, respectively, with EVAR having an advantage in postoperative mortality and morbidity.1,2 However, despite similar results regarding rupture and survival rates, these treatment concepts have fundamental differences. In traditional open surgical repair, the aneurysm is excluded from the arterial circulation under direct visualization. A surgical graft is sewn in with a tight suture at the proximal and distal ends of the aneurysm. Arterial side branches originating from the aneurysmal sac, such as the inferior mesenteric artery (IMA), lumbar arteries, and accessory renal arteries, are either surgically ligated or reanastomosed to the surgical graft if necessary. In any case, tight sutures and hemostasis must be achieved primarily. Unlike open repair, perigraft blood flow outside the endoprosthesis but inside the aneurysm sac may occur after EVAR. This phenomenon has been coined endoleak (EL) by White et al.3 In this chapter, the different types of ELs are described and the most rational imaging strategies for the diagnosis of ELs are discussed. The clinical significance and possible treatment options are also covered here. The term endoleak was introduced by White et al. in 1997 and is well accepted in the current literature.3 In earlier publications, terms such as perigraft flow, perigraft leak, or leakage were used to describe the same findings. According to White’s definition,3 endoleak is a condition associated with endoluminal vascular grafts, defined by the persistence of blood flow outside the lumen of the endoluminal graft but within an aneurysm sac or adjacent vascular segments being treated by the graft. Endoleak is due to incomplete sealing or exclusion of the aneurysm sac or vessel segment, as evidenced by imaging studies (e.g., contrast-enhanced computed tomography [CT] scanning, ultrasonography [US], angiography). White et al. also proposed the first systematic classification of ELs.3,4 This original classification was modified in a consensus conference of vascular surgeons and interventional radiologists, and the widely accepted classification was established.5,6 Primary ELs can be distinguished from secondary ELs according to the timing of development: • Primary ELs occur during the first 30 days after EVAR. These ELs usually develop immediately after EVAR, and the aneurysm was never actually completely excluded from the arterial circulation. • Secondary ELs occur later. Typically, the aneurysm has been excluded from the arterial circulation primarily. However, during follow-up, an EL not seen before is diagnosed. This type of EL is often caused by stent-graft distortion or migration. Another classification of EL is based on their source and differentiates five types (Table 50-1): TABLE 50-1 (Modified from Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002;35:1029–35; Baum RA, Stavropoulos SW, Fairman RM, et al. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol 2003;14:1111–7; and Parent FN, Meier GH, Godziachvili V, et al. The incidence and natural history of type I and II endoleak: a 5-year follow-up assessment with color duplex ultrasound scan. J Vasc Surg 2002;35:474–81.) • Type I ELs: In this type of EL, inflow occurs from the attachment sites of the endograft, so these ELs are also called attachment site endoleaks. If the leak occurs at the proximal attachment site, it is called a type IA EL (Fig. 50-1). If the leak is around the distal attachment site, it is a type IB leak. If aortouniiliac stent-grafts have been inserted and an EL occurs at the iliac occluder, it is a type IC EL. • Type II ELs: These ELs are also called reperfusion leaks. The aneurysm sac is partially perfused via arterial side branches originating from the aortic lumen. These side branches can be the IMA, lumbar arteries, or in rare cases, accessory renal arteries (Fig. 50-2). Direct arterial inflow into the sac occurs via this side branch perfusion despite tight sealing at the proximal and distal attachment sites. Typically, an inflow and outflow situation is present in type II ELs. Type II ELs can be further divided into simple single-vessel inflow and single-vessel outflow leaks (type IIA) and complex inflow and outflow vessel leaks, typically via multiple lumbar arteries (type IIB). • Type III ELs: Type III ELs are caused by graft failure. Typically these types of ELs occur during long-term follow-up and are due to some type of fatigue of the fabric material or fracture of the stent component, or both. The stent-graft is under constant mechanical microstress caused by the steady impact of arterial pulsation. Over time, stent wires may break, and holes can develop in the fabric material. Stent-graft distortion may also lead to a type III EL. Eventually, remodeling of the aneurysm may occur and lead to increased bending of the graft. Again, this can be a reason for wire fracture and perforation of the fabric material. With early modular stent-graft designs, junctional separations were observed and resulted in type III ELs (Figs. 50-3 and 50-4). In these stent-graft designs, the overlapping zones at the junctions were too short. This problem has been addressed in newer stent-graft designs simply by extending the junctional overlapping zones. Type III ELs can also be subcategorized. A type IIIA EL is present if the EL is caused by a midgraft hole. A type IIIB EL is a junctional leak, and a type IIIC EL is caused by any other type of graft failure. Generally speaking, with newer stent-graft designs, the incidence of type III ELs has decreased. However, long-term follow-up beyond a period of 10 years is needed to demonstrate whether modern stent-graft designs are durable enough. • Type IV ELs: These types of ELs are caused by graft porosity. The phenomenon of angiographic blush can be observed during the immediate postimplantation angiogram. Stent-graft designs differ in graft material composition, and immediately after implantation, some graft materials are temporarily slightly permeable. The degree of graft permeability is influenced by the degree of anticoagulation. This porosity is self-limiting and resolves within hours after EVAR. In most currently available stent-graft designs, graft material porosity has been reduced to a minimum, so type IV ELs are rarely a problem and have no real clinical relevance. The only important issue is that it can sometimes be difficult to differentiate a type IV arterial blush on the postimplantation angiogram from other clinically relevant forms of ELs. Some authors do not even consider a type IV EL to be a true EL. • Type V ELs: Type V ELs are summarized under the term endotension. These types of ELs were not described in White’s original 1997 classification but were added by the same authors 2 years later.7 A type V EL, or endotension, is present if the diameter of the aneurysmal sac increases during follow-up without a discernible EL. A subcategorization differentiates type VA, VB, VC, and VD ELs.6 Type VA EL is the actual endotension. Neither dual-phase contrast-enhanced CT nor any other imaging modality is capable of detecting an EL, and no EL has been shown in previous control studies, but the diameter of the sac increases. Type VB EL is present if the diameter of the sac increases and no EL is shown at a given follow-up CT, but a type I or II EL was shown on previous studies. Therefore, an EL might be still present but is not apparent on the given CT scan owing to flow dynamics. Type VC and VD ELs are surgically proven type I and III or type II ELs that have not been shown on CT or other imaging modalities. Development of an EL is the most common undesired event during or after EVAR. The reason a primary type I EL develops is improper patient selection in most cases. It has been shown that a short aneurysmal neck, severe angulation and calcification, and extensive thrombus lining of the neck are risk factors for a type I EL.8,9 If patients with these conditions are not considered for EVAR, primary type I ELs are rare events. Type III ELs are related to graft failure and are not under direct influence of the operator. These types of ELs were frequent with first- and second-generation stent-grafts.10 The incidence decreased significantly with third-generation devices. Type II ELs are not typical complications because their development can hardly be influenced by the operation; they are inherent phenomena of EVAR.3 Type IV ELs are not true ELs because they represent an angiographic feature without any clinical consequence.3 Type V ELs seem to be related to type II ELs and can just barely be called complications. In an analysis of 6787 patients from the registry database of the European Collaborating Groups on Stent-Graft Techniques for Abdominal Aortic Aneurysm Repair (EUROSTAR), the annual incidence of device-related ELs (i.e., type I and III ELs) was 6% and that of type II ELs was 5%.11 In this analysis, the authors showed a clear difference in performance of different stent-graft designs with regard to EL occurrence. Device-related ELs occur less frequently with second- and third-generation stent-grafts because they provide a better proximal and distal seal and better modular stability. There is clear evidence that ELs directly influence the clinical outcome of EVAR.12 It has been shown that type I and III ELs are significant risk factors for rupture after EVAR.13 The incidence of these types of ELs varies between 0% and 10%13 and strongly depends on patient selection. The situation is less clear for type II ELs. Gelfand et al. summarized the results of 10 EVAR trials involving a total of 2617 patients.14 The incidence of type II ELs was 6% to 17% at 30 days, 4.5% to 8% at 6 months, and 1% to 5% at 1 year. A EUROSTAR database analysis of 3595 patients showed significantly higher rates of secondary interventions in patients with type II ELs than in patients without ELs. However, conversion to open repair or post-EVAR rupture was not significantly associated with type II ELs.15 In contrast, rupture of aneurysms with a type II EL has been described, although this occurs rarely. The frequency of type V ELs is less well defined. Mennander et al. reported an incidence of 3.1% in a series of 160 patients; aneurysm rupture occurred in 3 of the 5 patients with endotension they followed. However, no significant intraperitoneal or retroperitoneal bleeding was observed.16 In many institutions, CT is still the most commonly used diagnostic imaging modality for post-EVAR patient follow-up. Typically, multiphase examinations are performed with multislice CT scanners, including an unenhanced, arterial, and delayed-phase scan.17–19 Unenhanced images may be helpful in differentiating small perigraft leaks from areas of calcified thrombus or metallic structures of the stent-graft.18 Typically the arterial-phase acquisition clearly shows perigraft flow if present. However, Rozenblit et al. showed that a delayed CT acquisition in addition to the arterial-phase scan can help detect additional ELs (Fig. 50-5). These authors also showed that the number of indeterminate findings can be reduced when an additional delayed-phase CT is acquired,19 so a triphasic CT acquisition protocol, including a delayed-phase scan acquired 50 seconds after injection of contrast material, has now been adopted in many institutions.20 In the case of a type I EL, direct communication of the parent vessel with the sac lumen may be depicted. Sometimes this communication can be displayed best in a multiformatted reconstruction (see Fig. 50-1). The diagnostic clue to a type II EL is seeing contrast-enhancing side branches with direct communication to the area of contrast enhancement in the sac (Fig. 50-6). In type III ELs, the EL is usually in direct communication with the stent-graft, and direct communication between the lumen of the stent-graft and sac can often be seen. Disintegration of the stent-graft and metallic strut fractures are strong indicators of a type III EL. Although the metallic skeleton of the stent-graft is typically evaluated by plain abdominal films, the same information can be provided by modern multislice CT scanners.21 Type IV ELs do not play a clinically significant role, as already mentioned. Although CT has been advocated as the gold standard for EL detection,19,22 it has several drawbacks. One is that a considerable amount of potentially nephrotoxic contrast medium is required. Depending on the multislice CT scan protocol, 80 to 120 mL of contrast medium per examination is used. Over a 5-year period, Azizzadeh et al.23 found impaired renal function in 83% of their 398 patients who underwent EVAR. Impaired renal function was defined according to the American Kidney Foundation as a glomerular filtration rate (GFR) of less than 90 mL/h as calculated with the Cockcroft equation. Interestingly, only 16.1% of patients had an abnormal serum creatinine level in this study. The authors found that patients with a preoperative GFR of less than 45 mL/h had significantly worse 48-month survival rates than patients with higher preoperative GFR values, although perioperative mortality was comparable. Based on these observations, it can be stated that repeated application of contrast material may have an adverse influence on the clinical course of at least some patients. Therefore, the authors conclude that periprocedural renal protection and the use of alternative contrast agents during follow-up might be beneficial in selected patients. The second drawback of multislice CT is radiation exposure. Although several technical features are implemented in modern CT scanners to reduce radiation exposure, multislice CT remains a radiologic examination responsible for more than two thirds of the total patient radiation dose associated with medical imaging.24 It is easy to understand that lifelong surveillance with at least annual CTs will lead to non-negligible radiation exposure. Based on an equation published by the International Commission for Radiation Protection, the lifetime risk for induction of cancer is 280 per million patients after a single abdominal CT scan.22 Assuming an observation period of 10 years with 12 CTs, this would roughly mean a risk of inducing cancer in about 1 patient in 300. In this very simplistic calculation, the radiation risk associated with single examinations is merely added; multiplication of risk as a result of accumulation of radiation damage has not been taken into consideration. Similarly, White and Macdonald estimate a total effective radiation exposure of 145 to 205 mSv for 70-year-old patients, including planning CT, EVAR, and surveillance CT at 1, 3, 6, and 12 months and yearly thereafter. In their calculation, this leads to a lifetime cancer risk of 0.42%, or 1 in 240. These numbers become more worrisome with decreasing patient age. For a 50-year-old patient, the cancer risk would be 0.73%, or 1 in 140.25 However, a mitigating factor is that we are dealing with an elderly patient population usually well above the age of 65, and it has been well documented that radiation risk is significantly lower in the elderly. Nevertheless, contrast medium load and radiation exposure are important concerns when serial CTs are performed for long-term follow-up of patients after EVAR. For this reason, two studies evaluated whether the commonly used multiphase CT protocol can be reduced by eliminating the early arterial phase,26 or the unenhanced or delayed phase, or both.27 The authors of the first study concluded that the arterial phase may not be necessary for routine EL detection, whereas the authors of the latter study found that the combination of unenhanced and arterial-phase CT affords the highest positive predictive value for detection of ELs after 1 month, and that the delayed phase does not significantly increase the sensitivity for detection of ELs, although ELs not seen on early-phase CT may be seen on delayed-phase CT.26,27 In another study, Bley et al. showed that serial volumetric analysis of nonenhanced CT may serve as an adequate screening test to identify ELs that cause a volumetric increase of more than 2%.28 Recently, dual-energy CT has been evaluated for EL detection after EVAR.29 With dual-energy CT, only one single delayed scan is acquired with a dual-source CT scanner. From this dataset, a virtual nonenhanced scan is calculated. Compared with a triphasic CT protocol, there was no significant difference in the numbers of ELs detected, but the radiation dose was up to 61% less. US is being used more frequently as an alternative to multiphase CT in patient follow-up after EVAR. It is less expensive, and no radiation or nephrotoxic contrast material is required (Fig. 50-7).30,31 The reliability of US in detecting ELs has been evaluated in numerous studies. In an early study, Sato et al. reported a 74% specificity and 97% sensitivity of color-coded US (ccUS) for detection of ELs.30 Better results were reported by Zannetti et al., who found an encouragingly high 91.7% sensitivity and 98.4% specificity of ccUS for EL detection, with CT serving as the standard of reference.31
Endoleaks
Classification, Diagnosis, and Treatment
Clinical Relevance
Definition
Classification of Endoleaks
Type I
Attachment site leak
A
Proximal
B
Distal
C
Iliac plug in aortouniiliac stent-grafts
Type II
A
Reperfusion leak
Simple single inflow/outflow
B
Leak
Complex side branch perfusion
Hyperdynamic on US
Quick wash-in
Hypodynamic on US
Slow wash-in
Type III
Device-related leak
A
Midgraft hole
B
Junctional separation
C
Any other leak caused by device failure
Type IV
Porosity leak
Type V
Endotension
A
Sac enlargement without demonstration of EL with any imaging modality
B
Sac enlargement, currently no EL discernible, but type I or II EL in previous examinations
C
Type I or III EL proven at surgery, not shown during follow-up imaging
D
Type II EL proven at surgery, not shown during follow-up imaging
Significance and Incidence of Endoleaks
Diagnosis of Endoleaks
Diagnosis of Endoleaks with Computed Tomography
Diagnosis of Endoleaks with Ultrasonography
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endoleaks: Classification, Diagnosis, and Treatment