Chapter Outline
Biliary Tract Infection and Inflammation
Complications of Endoscopic Retrograde Cholangiopancreatography
Since the implementation of endoscopic retrograde cholangiopancreatography (ERCP) into clinical practice in the late 1960s, ERCP has allowed exquisite display of the biliary tract and the pancreatic duct systems. The ducts were displayed with great spatial and contrast resolution; however, the ductal findings at times were also used as surrogates for parenchymal or local anatomic abnormalities.
Much of this information is now rightly being obtained noninvasively by the continued development of ultrasound, multidetector computed tomography (MDCT), and especially magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP). However, the ERCP examination has continued to change as well, now providing critical advancements in endoscopic therapeutic uses. The continued important presence of ERCP in clinical practice still demands radiology expertise for the interpretation of the findings of this examination.
Technique
Communication between the gastroenterologist and radiologist is critical, for both patient care and medical-legal reasons. With both services usually on busy schedules and frequently being physically separated, the radiologist can obtain information through the dedicated imaging technologist associated with the examination, closed-circuit television, and the gastroenterologist’s dictation from the electronic medical record system.
The examination should begin with an image centered over the patient’s right upper quadrant even before the patient is endoscoped. This image will allow the gastrointestinal service to appreciate any radiopaque material that might obscure ERCP imaging. This image will also display any calcifications related to the pancreatic and biliary systems that might be covered up with the injected 150 to 300 mg iodine/mL concentration of contrast medium that is typically used to opacify either system. This image and subsequent images are best obtained at 90 to 100 kVp, depending on the patient’s size. However, the fluoroscopic and imaging techniques are frequently adjusted automatically by the fluoroscopic unit.
The duct system is ideally imaged with the cannula placed initially at the level of the sphincter. However, the gastroenterologist will frequently feel more comfortable with the cannula advanced into the duct system for better purchase. Relatively slow injection rate with sequential imaging documentation is most useful for the interpreting radiologist. For example, biliary tract stones tend to congregate distally. A deep cannulation and rapid injection of contrast material can make display of these filling defects difficult.
The patient is typically placed in the prone oblique position for this examination. This results in flowing of the relatively heavier contrast agent away from the cannula, coursing “upstream.” With the injection, admixture artifacts may develop both at the cannula tip and at the site of inflow from the cystic duct into the biliary tract.
Also, because of the patient’s prone position, the left hepatic duct system will fill before the right. If the right duct system fills first, as is usual for T-tube cholangiography, in which the patient is typically supine, careful examination for left-sided obstruction is needed. A normal cystic duct and gallbladder should be filled during this examination. Images of early filling of the gallbladder may demonstrate the relatively heavy contrast agent’s beginning to outline the dependent gallstones in the gallbladder if it is present.
Imaging in multiple projections can be helpful in laying out normal or any abnormal anatomy. An attempt should be made to identify all of the central intrahepatic bile ducts. Lateral positioning of the patient can be helpful in distinguishing right from left ducts. In this position, the more anterior ducts belong to the left system ( Fig. 74-1 ).

Finally, delayed images can be helpful. Allowing some of the contrast agent to flow out of the duct system, greatly facilitated by supine positioning, can bring out findings lost in the dense contrast. Delayed imaging can also help confirm obstruction even if the ducts themselves do not appear to be dilated. A normal pancreatic duct should be drained of contrast material within 10 minutes from injection; the biliary tract, without a gallbladder, should take no more than 45 minutes.
Anatomy
Biliary Tract
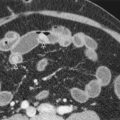
The biliary tract can be divided into the intrahepatic and extrahepatic systems. The intrahepatic biliary tract follows the arterial blood supply of the liver draining the eight hepatic segments. There is, however, moderate variability in the intrahepatic duct network; for example, the duct draining the right posterior segment is frequently aberrant ( Fig. 74-2 ). The intrahepatic ducts should appear smooth and gently taper as they course peripherally. The angles formed by these branches should be acute. Changes resulting in pruning of peripheral ducts, stiffening of the duct system, or deviation of the “flowing” appearance of this duct network should raise concern.

The extrahepatic ductal system actually begins with the right and left hepatic ducts, both of which have components outside the liver. The union of these two duct segments forms the common hepatic duct (CHD), which is approximately 2 to 4 cm in length.
The cystic duct joins the CHD at an acute angle, typically on the CHD’s right side. The cystic duct insertion may be anomalous. It may insert abnormally upstream in the CHD or even into the right posterior intrahepatic duct (see Fig. 74-2 ). It may also join the extrahepatic duct very distally or medially ( Fig. 74-3 ). This variant anatomy can lead to anatomic confusion and possible surgical mishap, or it may predispose to the development of complications, such as the Mirizzi syndrome.

The common bile duct (CBD) is formed with the union of the cystic duct and CHD. This 10-cm duct segment courses caudally and gently anteriorly when the patient is in the usual prone position during the ERCP examination (see Fig. 74-1 ). The combined CHD and CBD segments may be referred to as the common duct or extrahepatic biliary tree.
The distal CBD courses into the duodenal wall and into the major duodenal papilla, also named the papilla of Vater. This distal segment is usually smooth, but it may appear finely pleated or may contain a small diverticulum.
The distal CBD is usually met distally by the main pancreatic duct (MPD), the union of which forms a common channel, also known as the ampulla of Vater, of variable length ( Fig. 74-4A ). In approximately 10% to 20% of cases, each duct system enters the papilla and drains into the duodenum separately. This common channel varies in length from 2 to 15 mm but averages 5 mm.


These two ductal systems are wrapped in smooth muscle called the sphincter of Oddi ( Fig. 74-4B ), measuring 5 to 15 mm in length. This muscle segment has a constant, tonic basal pressure on which interval phasic contractions of approximately two or three times per minute are superimposed. These contractions can be appreciated at fluoroscopy during injection of contrast material. This phasic contraction can squeeze the ductal segments together to create the convex-upward appearance of a stone. This so-called pseudocalculus sign is reversed with the contraction’s end ( Fig. 74-5 ).

The diameter of the biliary tract should be measured at the CBD. Most authors suggest a maximal diameter, corrected for magnification with the known 12-mm-diameter of the therapeutic endoscope, to be less than 12 mm. With age, presumably related to degeneration of the duct’s elastic fiber network, the CBD may increase in diameter, resulting in a “floppy duct.” The normal CBD diameter of ERCP imaging will be greater than that of ultrasound, CT, and MRI, most likely related to the combination of active injection of contrast material and duct relaxation produced by conscious sedation used at ERCP.
Pancreatic Duct
The length of the MPD varies from 10 to 25 cm, but it averages 16 to 17 cm. It typically has an S-shaped configuration ( Fig. 74-6 ). The “toe” of the S courses horizontally from the sphincter, then ascends cranially. This segment drains the pancreatic head. The MPD then again turns into a horizontal direction corresponding to the “shoulder,” running anterior to the superior mesenteric artery and its vein. The transition from the ascending to the horizontal segment is designated the neck. The remainder of the pancreas is divided between the body and the tail, either with the left side of the spine being the transition point or by simply dividing the duct from the neck to the end of the tail in half.

The neck of the MPD may have a subtle, circumferential narrowing without upstream dilation. This normal variant is thought to represent the union of the embryonic dorsal and ventral ducts. Toward the upstream segment of the MPD, there is often bifurcation of the duct as it nears its termination. Anatomically, the tail is proximal and the head is considered distal, although some use upstream to describe anatomy toward the tail and downstream to refer to the anatomy coursing toward the pancreatic head.
The MPD results from the union of the embryologic ventral duct, Wirsung, and the dorsal duct, Santorini. The MPD has a smooth, gentle narrowing from the head to the tail. The maximum diameter, again magnification corrected, at the head is less than 6 mm. The ERCP normal values will again be less than those related to ultrasound, CT, and MRI. With age, the MPD may normally develop a more “stiff” appearance with some subtle ductal irregularity.
There is no “normal” side branch appearance on ERCP. Side branches may have a fine, slender appearance ( Fig. 74-6 ), or they may be wider in diameter. However, a given patient’s side branches should be uniform in appearance and should have a smooth, gentle tapering configuration as they head into the pancreatic parenchyma. Display of the side branch system, as well as of the MPD, to assess for various abnormalities is infrequent in today’s practice with the other imaging methods now available and because the risk of producing pancreatitis is relatively great with MPD injection.
Biliary Tract Neoplasia
Malignant neoplasms involving the biliary tract, both intrahepatic and extrahepatic, usually are manifested as stricture disease and less commonly as filling defects. The strictured biliary tract segment can be the result of direct mural involvement by the tumor or of compression or infiltration from adjacent diseased lymph nodes or hepatic parenchymal tumor. Unfortunately, benign disease involving the biliary tract also frequently results in a strictured appearance ( Box 74-1 ). The different causes of the biliary stricture must be differentiated for appropriate management.
Infectious, Inflammatory, Miscellaneous
Postsurgical
Pancreatitis
Primary sclerosing cholangitis
Immunoglobulin G4–related disease
AIDS cholangiopathy
Recurrent pyogenic cholangitis
Post–liver transplantation
Sphincter of Oddi dysfunction
Cirrhosis
Intra-arterial chemotherapy
Primary biliary cirrhosis
Inflammatory pseudotumor (malignant masquerade)
Cystic fibrosis
Radiation therapy
Post-traumatic
Post–percutaneous ablation of hepatic tumor
Sarcoidosis
Tuberculosis
Eosinophilic cholangiopathy
Portal biliopathy
Secondary sclerosing cholangitis
Duodenal diverticula
Adjacent abscess or pseudocyst
Neoplastic: Malignant, Aggressive
Pancreatic cancer
Cholangiocarcinoma
Biliary papilloma or papillomatosis
Gallbladder carcinoma
Periampullary tumors
Hepatocellular carcinoma
Metastatic disease
Lymphoma
Biliary cystadenoma or cystadenocarcinoma
Neoplastic: Benign, Nonaggressive
Granular cell tumor
Morphologic appearance can be of some help in this differentiation. Malignant strictures tend to be long (≥1.5 cm), have an irregular, eccentric mural surface, and have a short (<1 cm) transitional zone with a nodular or shouldered appearance. The addition of contrast-enhanced MDCT or MRI can add criteria for a malignant appearance with increasing mural thickness, irregularity of the external mural border, and mural enhancement.
The cholangiographic appearance in identifying a malignant stricture is only approximately 70% to 85% for sensitivity, specificity, positive predictive value, and accuracy, so the added benefit of tissue sampling at ERCP can be important. Cells and tissue can be acquired by various methods: aspirated bile for cytology, brush cytology, fine-needle aspiration of the stricture for cytology, endobiliary forceps biopsy, and even analysis of material clinging to a retrieved plastic stent. Multiple types of acquisition methods will increase return. The sensitivity of either brush cytology or biopsy is approximately 56%, but their combined use improves the return to 73%. It has now been reported that the previous practice of sampling after stricture dilation does not increase the return on sampling. These results can be compared with the use of fine-needle aspiration at endoscopic ultrasound (EUS), which has a high return, at least 80%, for distal malignant strictures from pancreatic cancer or cholangiocarcinoma.
Endoscopic techniques are also useful for stent placement in lower duct malignant strictures. The clinical views of what type of stent to use and when to use stents in malignant strictures are continuing to evolve.
Pancreatic cancer is the most common cause of malignant biliary tract obstruction. Cholangiocarcinoma and gallbladder cancer are the most common primary bile duct tumors. A group of four different types of tumors, including pancreatic cancer and distal cholangiocarcinoma, can occur at the ampullary site to also cause bile duct obstruction, called periampullary tumors. Metastatic disease also is a common cause of malignant biliary tract obstruction, with gastrointestinal tract tumors, hepatocellular carcinoma, and lymphoma being common causes.
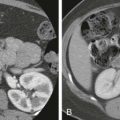
Pancreatic cancer involvement of the biliary tract is usually seen in the distal CBD segment, the intrapancreatic portion of the CBD ( Fig. 74-7A, B ). The length of bile duct stricture depends on both the tumor size within the pancreatic head and the nearness of the bile duct to the tumor’s center. The transition of the strictured segment is usually rapid, with a nodular or shouldered appearance. However, as discussed before, pancreatic carcinoma can involve the proximal extrahepatic biliary tree from adjacent peribiliary lymph node involvement, and it can also involve the intrahepatic ducts if there is hepatic metastatic disease ( Fig. 74-7C, D ).

Cholangiocarcinoma can be intrahepatic or extrahepatic in location. It is classified into one of three types: mass forming, periductal infiltrating, and intraductal growth. The mass-forming type of cholangiocarcinoma arises from small intrahepatic ducts. The carcinoma forms a hepatic parenchymal mass; MDCT and MRI are the most appropriate modalities for its detection and assessment for extent of disease. Cholangiography does not play a significant role in imaging.
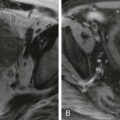
The tumor associated with the periductal infiltrating cholangiocarcinoma grows within the wall of the biliary tract, causing mural thickening with an irregular ( Fig. 74-8A ) or smooth ( Fig. 74-8B ) stricture. It spreads in a submucosal manner and tends to infiltrate the periductal anatomy. This tumor frequently begins in the hilar region, where it is referred to as a Klatskin tumor. The tumor can grow upstream or downstream. Unfortunately, it also tends to extend into the periductal and perineural tissues as well as into the lymphatics, where MDCT or MRI may better define the extramural disease. ERCP does have a place if resection is anticipated ( Fig. 74-8C ). Cholangiography can be used to better define the biliary tract anatomy involvement by the Bismuth classification ( Fig. 74-9 ). The Bismuth classification is used to evaluate proximal biliary tract resection of strictures or obstructions of various causes. ERCP can provide important information about the local anatomy through its exquisite spatial display of the duct system.


At ERCP, the early periductal infiltrating type of cholangiocarcinoma can be manifested as a relatively short, smooth appearance to simulate a benign stricture. However, because cholangiocarcinoma is usually appreciated later in the disease process, this stricture tends to be longer and more irregular in appearance.
The much less common type of cholangiocarcinoma, the intraductal growing type (also referred to as papillary cholangiocarcinoma), is the most likely type of cholangiocarcinoma to be manifested relatively early in its course. It tends to spread superficially and not invade the bile duct wall. It has a better prognosis compared with the other types of cholangiocarcinoma. At ERCP, this tumor may be a well-defined, smooth, intraluminal papillary protrusion (see Fig. 74-8C ); but there can be multiple papillary projections, and some of these papillary projections may have an irregular surface. The intraductal growing type of cholangiocarcinoma may cause obstruction of the upstream ductal system without clear definition of the tumor itself. This type of cholangiocarcinoma can also produce mucin to cause biliary dilation. There is a complex relationship between the intraductal growing type of cholangiocarcinoma, biliary papilloma or papillomatosis ( Fig. 74-10 ), and intraductal papillary mucinous neoplasm of the bile duct ( Fig. 74-11 ), all of which can be seen as a papillary projection on cholangiography.


Gallbladder carcinoma is the other primary biliary tract malignant neoplasm. Local disease from this primary may spread to the mid or proximal extrahepatic biliary tree by direct extension along the cystic duct, by lymphatics, or by enlarged peribiliary lymph nodes. The stricture may extend to the hilar bifurcation or into the right or left hepatic duct system. The intrahepatic ducts may also be compromised by the cancer’s predilection to invade the adjacent hepatic parenchyma or from metastatic deposits within the liver, which can displace, infiltrate, or obstruct the intrahepatic duct system.
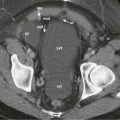
At ERCP, the cystic duct may not fill or only partially fill. The mid to proximal duct can be splayed, be diffusely narrowed, or demonstrate an irregular stricture ( Fig. 74-12 ). Some mass effect, stricture, or obstruction can also be related to enlarged hepatobiliary lymph nodes.

Distal CBD obstruction, and potentially downstream MPD obstruction, can be caused by a variety of tumors that can occur at the papilla of Vater. Because of the difficulty in imaging, endoscopy, surgery, and even histology to separate these tumors, these tumors are combined under the heading of periampullary tumors. These tumors can be either benign or malignant. Benign tumors in this area are less common and include the ampullary adenoma, local geographic benign tumors, and carcinoid.
Periampullary cancer refer to cancers from one of four structures: the pancreatic head, distal bile duct, duodenum, and ampulla. There are other, rare malignant neoplasms that can occur here as well, such as metastatic disease and lymphoma. Even though these cancers share a similar location and clinical presentation, the underlying histologic type can dictate prognosis. The ampullary and duodenal cancers have the best prognosis, whereas the distal bile duct and pancreatic cancers have the worst.
Although some authors suggest that contrast-enhanced MDCT with three-dimensional reconstruction is the preoperative imaging examination of choice for the periampullary tumor, CT can have difficulty in both the visualization and the accurate assessment of local disease. Biliary or pancreatic duct dilation may be visualized but the underlying tumor not appreciated.
ERCP can be of great help by directly visualizing the periampullary tumor at the time of endoscopy and display the possible intraductal involvement of the tumor during ductal injection of contrast material ( Fig. 74-13 ). Tissue retrieval can also be obtained as well as stenting, if necessary, and even local endoscopic resection in the appropriate case. However, EUS and even transpapillary intraductal ultrasound are frequently used by endoscopists for better assessment of local tissue involvement and possible local lymph node spread. EUS can also be used for fine-needle aspiration of the tumor for histologic diagnosis. A periampullary tumor may not have an intraluminal component. The cholangiogram or pancreatogram may demonstrate only a narrowing without an intraluminal mass. In these cases, tissue retrieval is critical.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree